Age-related changes to adipose tissue and peripheral neuropathy in genetically diverse HET3 mice differ by sex and are not mitigated by rapamycin longevity treatment
- PMID: 36798047
- PMCID: PMC10086534
- DOI: 10.1111/acel.13784
Age-related changes to adipose tissue and peripheral neuropathy in genetically diverse HET3 mice differ by sex and are not mitigated by rapamycin longevity treatment
Abstract
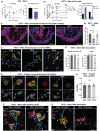
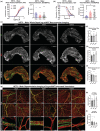
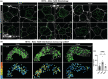
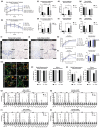
Neural communication between the brain and adipose tissues regulates energy expenditure and metabolism through modulation of adipose tissue functions. We have recently demonstrated that under pathophysiological conditions (obesity, diabetes, and aging), total subcutaneous white adipose tissue (scWAT) innervation is decreased ('adipose neuropathy'). With advanced age in the C57BL/6J mouse, small fiber peripheral nerve endings in adipose tissue die back, resulting in reduced contact with adipose-resident blood vessels and other cells. This vascular neuropathy and parenchymal neuropathy together likely pose a physiological challenge for tissue function. In the current work, we used the genetically diverse HET3 mouse model to investigate the incidence of peripheral neuropathy and adipose tissue dysregulation across several ages in both male and female mice. We also investigated the anti-aging treatment rapamycin, an mTOR inhibitor, as a means to prevent or reduce adipose neuropathy. We found that HET3 mice displayed a reduced neuropathy phenotype compared to inbred C56BL/6 J mice, indicating genetic contributions to this aging phenotype. Compared to female HET3 mice, male HET3 mice had worse neuropathic phenotypes by 62 weeks of age. Female HET3 mice appeared to have increased protection from neuropathy until advanced age (126 weeks), after reproductive senescence. We found that rapamycin overall had little impact on neuropathy measures, and actually worsened adipose tissue inflammation and fibrosis. Despite its success as a longevity treatment in mice, higher doses and longer delivery paradigms for rapamycin may lead to a disconnect between life span and beneficial health outcomes.
Keywords: HET3 mice; adipose tissue; aging; collagen; fibrosis; neuromuscular junction (NMJ); peripheral neuropathy; rapamycin; sex differences.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Life-span Extension Drug Interventions Affect Adipose Tissue Inflammation in Aging.J Gerontol A Biol Sci Med Sci. 2020 Jan 1;75(1):89-98. doi: 10.1093/gerona/glz177. J Gerontol A Biol Sci Med Sci. 2020. PMID: 31353414 Free PMC article.
-
Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive.Aging Cell. 2013 Aug;12(4):712-8. doi: 10.1111/acel.12097. Epub 2013 Jun 7. Aging Cell. 2013. PMID: 23648089 Free PMC article.
-
Neuropathy and neural plasticity in the subcutaneous white adipose depot.PLoS One. 2019 Sep 11;14(9):e0221766. doi: 10.1371/journal.pone.0221766. eCollection 2019. PLoS One. 2019. PMID: 31509546 Free PMC article.
-
Role of leptin resistance in the development of obesity in older patients.Clin Interv Aging. 2013;8:829-44. doi: 10.2147/CIA.S36367. Epub 2013 Jul 4. Clin Interv Aging. 2013. PMID: 23869170 Free PMC article. Review.
-
Fat tissue and long life.Obes Facts. 2008;1(4):176-82. doi: 10.1159/000145930. Epub 2008 Aug 14. Obes Facts. 2008. PMID: 20054178 Free PMC article. Review.
Cited by
-
Genetic diversity leads to differential inflammatory responses to cigarette smoke in mice.Physiol Rep. 2025 Jan;13(2):e70199. doi: 10.14814/phy2.70199. Physiol Rep. 2025. PMID: 39844449 Free PMC article.
-
The Sympathetic Innervation of Adipose Tissues: Regulation, Functions, and Plasticity.Compr Physiol. 2023 Jun 26;13(3):4985-5021. doi: 10.1002/cphy.c220030. Compr Physiol. 2023. PMID: 37358505 Free PMC article.
-
Gene therapy approaches for obesity-induced adipose neuropathy: Device-targeted AAV-mediated neurotrophic factor delivery to adipocytes in subcutaneous adipose.Mol Ther. 2024 May 1;32(5):1407-1424. doi: 10.1016/j.ymthe.2024.02.035. Epub 2024 Mar 1. Mol Ther. 2024. PMID: 38429927 Free PMC article.
-
Adipose tissue as a linchpin of organismal ageing.Nat Metab. 2024 May;6(5):793-807. doi: 10.1038/s42255-024-01046-3. Epub 2024 May 23. Nat Metab. 2024. PMID: 38783156 Free PMC article. Review.
-
New perspectives on polycystic ovary syndrome: hypothalamic-sympathetic-adipose tissue interaction.J Ovarian Res. 2025 Jul 4;18(1):145. doi: 10.1186/s13048-025-01724-0. J Ovarian Res. 2025. PMID: 40615863 Free PMC article. Review.
References
-
- Arriola Apelo, S. I. , Neuman, J. C. , Baar, E. L. , Syed, F. A. , Cummings, N. E. , Brar, H. K. , Pumper, C. P. , Kimple, M. E. , & Lamming, D. W. (2016). Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell, 15(1), 28–38. 10.1111/acel.12405 - DOI - PMC - PubMed
-
- Blaszkiewicz, M. , Willows, J. W. , Dubois, A. L. , Waible, S. , DiBello, K. , Lyons, L. L. , Johnson, C. P. , Paradie, E. , Banks, N. , Motyl, K. , Michael, M. , Harrison, B. , & Townsend, K. L. (2019). Neuropathy and neural plasticity in the subcutaneous white adipose depot. PLoS One, 14(9), e0221766. 10.1371/journal.pone.0221766 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

