High-dose Intravenous Vitamin C in Early Stages of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Double-blind, Randomized, Controlled Clinical Trial
- PMID: 36798102
- PMCID: PMC9926917
- DOI: 10.4103/jrpp.jrpp_30_22
High-dose Intravenous Vitamin C in Early Stages of Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Double-blind, Randomized, Controlled Clinical Trial
Abstract
Objective: Based on previous studies in the sepsis population, Vitamin C could prevent injuries when administered in high doses and before the damage is established. This study aimed to evaluate the protective potentials of high-dose Vitamin C in the progression of coronavirus disease 2019 (COVID-19).
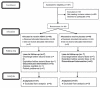
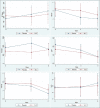
Methods: A double-blind, placebo-controlled clinical trial was conducted. Patients with moderate-to-severe disease severity based on the World Health Organization definition were enrolled and received 12 g/d Vitamin C (high-dose intravenous Vitamin C [HDIVC]) or placebo for 4 days. Sequential Organ Failure Assessment (SOFA) score as a primary outcome, National Early Warning Score, Ordinal Scale of Clinical Improvement, and cytokine storm biomarkers were recorded on days 0, 3, and 5. Survival was also assessed on day 28 after enrollment.
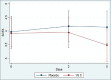
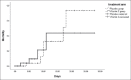
Findings: Seventy-four patients (37 patients in each group) were enrolled from April 5, 2020, to November 19, 2020, and all patients completed follow-up. A lower increase in SOFA score during the first 3 days of treatment (+0.026 vs. +0.204) and a higher decrease in this parameter in the last 2 days (-0.462 vs. -0.036) were observed in the treatment group. However, these differences did not reach a significance level (P = 0.57 and 0.12, respectively). Other indices of clinical and biological improvement, length of hospitalization, and intensive care unit admission days were the same between the two groups. Treatment did not affect the 28-day mortality.
Conclusion: Among patients with moderate-to-severe disease of COVID-19, the use of HDIVC plus standard care resulted in no significant difference in SOFA score or 28-day mortality compared to the standard care alone.
Keywords: Coronavirus disease 2019; high-dose Vitamin C; inflammation; severe acute respiratory syndrome coronavirus 2.
Copyright: © 2022 Journal of Research in Pharmacy Practice.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Pilot trial of high-dose vitamin C in critically ill COVID-19 patients.Ann Intensive Care. 2021 Jan 9;11(1):5. doi: 10.1186/s13613-020-00792-3. Ann Intensive Care. 2021. PMID: 33420963 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
A Phase 3 Open-label, Randomized, Controlled Study to Evaluate the Efficacy and Safety of Intravenously Administered Ravulizumab Compared with Best Supportive Care in Patients with COVID-19 Severe Pneumonia, Acute Lung Injury, or Acute Respiratory Distress Syndrome: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jul 13;21(1):639. doi: 10.1186/s13063-020-04548-z. Trials. 2020. PMID: 32660611 Free PMC article.
-
Vitamin D supplementation for the treatment of COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 24;5(5):CD015043. doi: 10.1002/14651858.CD015043. Cochrane Database Syst Rev. 2021. PMID: 34029377 Free PMC article.
-
The effectiveness of high-dose intravenous vitamin C for patients with coronavirus disease 2019: A systematic review and meta-analysis.Complement Ther Med. 2022 Mar;64:102797. doi: 10.1016/j.ctim.2021.102797. Epub 2021 Dec 22. Complement Ther Med. 2022. PMID: 34953366 Free PMC article.
Cited by
-
Vitamin D: A Nutraceutical Supplement at the Crossroad Between Respiratory Infections and COVID-19.Int J Mol Sci. 2025 Mar 12;26(6):2550. doi: 10.3390/ijms26062550. Int J Mol Sci. 2025. PMID: 40141190 Free PMC article. Review.
-
Role of Antioxidant Therapy in the Treatment and Prognosis of COVID-19: A Systematic Review and Meta-analysis of Randomized Controlled Trials.Curr Dev Nutr. 2024 Mar 24;8(5):102145. doi: 10.1016/j.cdnut.2024.102145. eCollection 2024 May. Curr Dev Nutr. 2024. PMID: 38693966 Free PMC article.
-
Vitamin D Supplementation in Neonatal and Infant MIS-C Following COVID-19 Infection.Int J Mol Sci. 2024 Mar 27;25(7):3712. doi: 10.3390/ijms25073712. Int J Mol Sci. 2024. PMID: 38612523 Free PMC article. Review.
-
The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials.Inflammopharmacology. 2023 Dec;31(6):3357-3362. doi: 10.1007/s10787-023-01200-5. Epub 2023 Apr 18. Inflammopharmacology. 2023. PMID: 37071316 Free PMC article.
-
The Efficacy of Multivitamin, Vitamin A, Vitamin B, Vitamin C, and Vitamin D Supplements in the Prevention and Management of COVID-19 and Long-COVID: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials.Nutrients. 2024 Apr 29;16(9):1345. doi: 10.3390/nu16091345. Nutrients. 2024. PMID: 38732592 Free PMC article.
References
-
- Clinical management of COVID-19: Living guideline. 2022 WHO/2019-nCoV/Clinical/2022.1. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
