Novel Investigational Anti-SARS-CoV-2 Agent Ensitrelvir "S-217622": A Very Promising Potential Universal Broad-Spectrum Antiviral at the Therapeutic Frontline of Coronavirus Species
- PMID: 36798145
- PMCID: PMC9897045
- DOI: 10.1021/acsomega.2c03881
Novel Investigational Anti-SARS-CoV-2 Agent Ensitrelvir "S-217622": A Very Promising Potential Universal Broad-Spectrum Antiviral at the Therapeutic Frontline of Coronavirus Species
Abstract
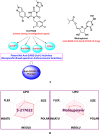
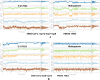
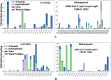
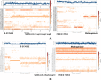
Lately, nitrogenous heterocyclic antivirals, such as nucleoside-like compounds, oxadiazoles, thiadiazoles, triazoles, quinolines, and isoquinolines, topped the therapeutic scene as promising agents of choice for the treatment of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and their accompanying ailment, the coronavirus disease 2019 (COVID-19). At the same time, the continuous emergence of new strains of SARS-CoV-2, like the Omicron variant and its multiple sublineages, resulted in a new defiance in the enduring COVID-19 battle. Ensitrelvir (S-217622) is a newly discovered orally active noncovalent nonpeptidic agent with potential strong broad-spectrum anticoronaviral activities, exhibiting promising nanomolar potencies against the different SARS-CoV-2 variants. S-217622 effectively and nonspecifically hits the main protease (Mpro) enzyme of a broad scope of coronaviruses. Herein, in the present computational/biological study, we tried to extend these previous findings to prove the universal activities of this investigational agent against any coronavirus, irrespective of its type, through synchronously acting on most of its main unchanged replication enzymes/proteins, including (in addition to the Mpro), e.g., the highly conserved RNA-dependent RNA polymerase (RdRp) and 3'-to-5' exoribonuclease (ExoN). Biochemical evaluation proved, using the in vitro anti-RdRp/ExoN bioassay, that S-217622 can potently inhibit the replication of coronaviruses, including the new virulent strains of SARS-CoV-2, with extremely minute in vitro anti-RdRp and anti-RdRp/ExoN half-maximal effective concentration (EC50) values of 0.17 and 0.27 μM, respectively, transcending the anti-COVID-19 drug molnupiravir. The preliminary in silico results greatly supported these biochemical results, proposing that the S-217622 molecule strongly and stabilizingly strikes the key catalytic pockets of the SARS-CoV-2 RdRp's and ExoN's principal active sites predictably via the nucleoside analogism mode of anti-RNA action (since the S-217622 molecule can be considered as a uridine analog). Moreover, the idealistic druglikeness and pharmacokinetic characteristics of S-217622 make it ready for pharmaceutical formulation with the expected very good clinical behavior as a drug for the infections caused by coronaviruses, e.g., COVID-19. To cut it short, the current critical findings of this extension work significantly potentiate and extend the S-217622's previous in vitro/in vivo (preclinical) results since they showed that the striking inhibitory activities of this novel anti-SARS-CoV-2 agent on the Mpro could be extended to other replication enzymes like RdRp and ExoN, unveiling the possible universal use of the compound against the next versions of the virus (i.e., disclosing the nonspecific anticoronaviral properties of this compound against almost any coronavirus strain), e.g., SARS-CoV-3, and encouraging us to rapidly start the compound's vast clinical anti-COVID-19 evaluations.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Potent Dual Polymerase/Exonuclease Inhibitory Activities of Antioxidant Aminothiadiazoles Against the COVID-19 Omicron Virus: A Promising In Silico/In Vitro Repositioning Research Study.Mol Biotechnol. 2024 Apr;66(4):592-611. doi: 10.1007/s12033-022-00551-8. Epub 2023 Jan 24. Mol Biotechnol. 2024. PMID: 36690820 Free PMC article.
-
A Series of Adenosine Analogs as the First Efficacious Anti-SARS-CoV-2 Drugs against the B.1.1.529.4 Lineage: A Preclinical Repurposing Research Study.ChemistrySelect. 2022 Dec 13;7(46):e202201912. doi: 10.1002/slct.202201912. Epub 2022 Dec 8. ChemistrySelect. 2022. PMID: 36718467 Free PMC article.
-
Promising Experimental Anti-SARS-CoV-2 Agent "SLL-0197800": The Prospective Universal Inhibitory Properties against the Coming Versions of the Coronavirus.ACS Omega. 2023 Sep 18;8(39):35538-35554. doi: 10.1021/acsomega.2c08073. eCollection 2023 Oct 3. ACS Omega. 2023. Retraction in: ACS Omega. 2025 Mar 31;10(14):14554. doi: 10.1021/acsomega.5c01901. PMID: 37810715 Free PMC article. Retracted.
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
-
SARS-CoV-2 Omicron Variant in Medicinal Chemistry Research.Curr Top Med Chem. 2023;23(17):1625-1639. doi: 10.2174/1568026623666230411095417. Curr Top Med Chem. 2023. PMID: 37055893 Review.
Cited by
-
Main and papain-like proteases as prospective targets for pharmacological treatment of coronavirus SARS-CoV-2.RSC Adv. 2023 Dec 6;13(50):35500-35524. doi: 10.1039/d3ra06479d. eCollection 2023 Nov 30. RSC Adv. 2023. PMID: 38077980 Free PMC article. Review.
-
Identification of broad-spectrum Mpro inhibitors: a focus on high-risk coronaviruses and conserved interactions.J Enzyme Inhib Med Chem. 2025 Dec;40(1):2503961. doi: 10.1080/14756366.2025.2503961. Epub 2025 May 21. J Enzyme Inhib Med Chem. 2025. PMID: 40396609 Free PMC article.
-
Antrodia cinnamomea May Interfere with the Interaction Between ACE2 and SARS-CoV-2 Spike Protein in vitro and Reduces Lung Inflammation in a Hamster Model of COVID-19.J Inflamm Res. 2023 Oct 26;16:4867-4884. doi: 10.2147/JIR.S431222. eCollection 2023. J Inflamm Res. 2023. PMID: 37908202 Free PMC article.
-
Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials.Front Pharmacol. 2023 Aug 24;14:1228548. doi: 10.3389/fphar.2023.1228548. eCollection 2023. Front Pharmacol. 2023. PMID: 37693894 Free PMC article.
-
Discovery of Hybrid Thiouracil-Coumarin Conjugates as Potential Novel Anti-SARS-CoV-2 Agents Targeting the Virus's Polymerase "RdRp" as a Confirmed Interacting Biomolecule.ACS Omega. 2023 Jul 14;8(30):27056-27066. doi: 10.1021/acsomega.3c02079. eCollection 2023 Aug 1. ACS Omega. 2023. PMID: 37546653 Free PMC article.
References
-
- Kaur H.; Sarma P.; Bhattacharyya A.; Sharma S.; Chhimpa N.; Prajapat M.; Prakash A.; Kumar S.; Singh A.; Singh R.; Avti P.; Thota P.; Medhi B. Efficacy and safety of dihydroorotate dehydrogenase (DHODH) inhibitors ″leflunomide″ and ″teriflunomide″ in Covid-19: A narrative review. Eur. J. Pharmacol. 2021, 906, 17423310.1016/j.ejphar.2021.174233. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

