This is a preprint.
HiExM: high-throughput expansion microscopy enables scalable super-resolution imaging
- PMID: 36798312
- PMCID: PMC9934540
- DOI: 10.1101/2023.02.07.527509
HiExM: high-throughput expansion microscopy enables scalable super-resolution imaging
Update in
-
High-throughput expansion microscopy enables scalable super-resolution imaging.Elife. 2024 Nov 26;13:RP96025. doi: 10.7554/eLife.96025. Elife. 2024. PMID: 39589396 Free PMC article.
Abstract
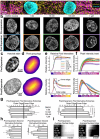
Expansion microscopy (ExM) enables nanoscale imaging using a standard confocal microscope through the physical, isotropic expansion of fixed immunolabeled specimens. ExM is widely employed to image proteins, nucleic acids, and lipid membranes in single cells; however, current methods limit the number of samples that can be processed simultaneously. We developed High-throughput Expansion Microscopy (HiExM), a robust platform that enables expansion microscopy of cells cultured in a standard 96-well plate. Our method enables ~4.2x expansion of cells within individual wells, across multiple wells, and between plates. We also demonstrate that HiExM can be combined with high-throughput confocal imaging platforms to greatly improve the ease and scalability of image acquisition. As an example, we analyzed the effects of doxorubicin, a known cardiotoxic agent, on human cardiomyocytes (CMs) as measured by Hoechst signal across the nucleus. We show a dose dependent effect on nuclear DNA that is not observed in unexpanded CMs, suggesting that HiExM improves the detection of cellular phenotypes in response to drug treatment. Our method broadens the application of ExM as a tool for scalable super-resolution imaging in biological research applications.
Keywords: expansion microscopy; high-throughput; nanoscopy; super-resolution imaging.
Figures


References
-
- Gustafsson M. G. L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy: SHORT COMMUNICATION. J. Microsc. 198, 82–87 (2000). - PubMed
-
- Hell S. W. & Wichmann J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780 (1994). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
