This is a preprint.
Modulation of GPR133 (ADGRD1) Signaling by its Intracellular Interaction Partner Extended Synaptotagmin 1 (ESYT1)
- PMID: 36798364
- PMCID: PMC9934660
- DOI: 10.1101/2023.02.09.527921
Modulation of GPR133 (ADGRD1) Signaling by its Intracellular Interaction Partner Extended Synaptotagmin 1 (ESYT1)
Update in
-
Modulation of GPR133 (ADGRD1) signaling by its intracellular interaction partner extended synaptotagmin 1.Cell Rep. 2024 May 28;43(5):114229. doi: 10.1016/j.celrep.2024.114229. Epub 2024 May 16. Cell Rep. 2024. PMID: 38758649 Free PMC article.
Abstract
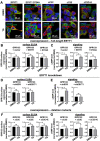
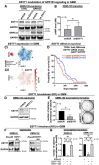
GPR133 (ADGRD1) is an adhesion G protein-coupled receptor that signals through Gαs and is required for growth of glioblastoma (GBM), an aggressive brain malignancy. The regulation of GPR133 signaling is incompletely understood. Here, we use proximity biotinylation proteomics to identify ESYT1, a Ca2+-dependent mediator of endoplasmic reticulum-plasma membrane bridge formation, as an intracellular interactor of GPR133. ESYT1 knockdown or knockout increases GPR133 signaling, while its overexpression has the opposite effect, without altering GPR133 levels in the plasma membrane. The GPR133-ESYT1 interaction requires the Ca2+-sensing C2C domain of ESYT1. Thapsigargin-mediated increases in cytosolic Ca2+ relieve signaling-suppressive effects of ESYT1 by promoting ESYT1-GPR133 dissociation. ESYT1 knockdown or knockout in GBM impairs tumor growth in vitro, suggesting functions of ESYT1 beyond the interaction with GPR133. Our findings suggest a novel mechanism for modulation of GPR133 signaling by increased cytosolic Ca2+, which reduces the signaling-suppressive interaction between GPR133 and ESYT1 to raise cAMP levels.
Keywords: ESYT1; GPR133; adhesion G protein-coupled receptor; cAMP; calcium; extended synaptotagmin.
Conflict of interest statement
Disclosures and Competing Interests DGP and NYU Grossman School of Medicine own an EU and Hong Kong patent titled “Method for treating high-grade gliomas” on the use of GPR133 as a treatment target in glioma. DGP has received consultant fees from Tocagen, Synaptive Medical, Monteris, Robeaute and Advantis.
Figures






References
-
- Bayin N.S., Frenster J.D., Kane J.R., Rubenstein J., Modrek A.S., Baitalmal R., Dolgalev I., Rudzenski K., Scarabottolo L., Crespi D., et al. (2016). GPR133 (ADGRD1), an adhesion G-protein-coupled receptor, is necessary for glioblastoma growth. Oncogenesis 5, e263. 10.1038/oncsis.2016.63. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
