This is a preprint.
Somatotopic organization among parallel sensory pathways that promote a grooming sequence in Drosophila
- PMID: 36798384
- PMCID: PMC9934617
- DOI: 10.1101/2023.02.11.528119
Somatotopic organization among parallel sensory pathways that promote a grooming sequence in Drosophila
Update in
-
Somatotopic organization among parallel sensory pathways that promote a grooming sequence in Drosophila.Elife. 2024 Apr 18;12:RP87602. doi: 10.7554/eLife.87602. Elife. 2024. PMID: 38634460 Free PMC article.
Abstract
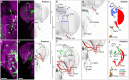
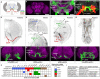
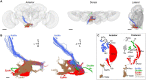
Mechanosensory neurons located across the body surface respond to tactile stimuli and elicit diverse behavioral responses, from relatively simple stimulus location-aimed movements to complex movement sequences. How mechanosensory neurons and their postsynaptic circuits influence such diverse behaviors remains unclear. We previously discovered that Drosophila perform a body location-prioritized grooming sequence when mechanosensory neurons at different locations on the head and body are simultaneously stimulated by dust (Hampel et al., 2017; Seeds et al., 2014). Here, we identify nearly all mechanosensory neurons on the Drosophila head that individually elicit aimed grooming of specific head locations, while collectively eliciting a whole head grooming sequence. Different tracing methods were used to reconstruct the projections of these neurons from different locations on the head to their distinct arborizations in the brain. This provides the first synaptic resolution somatotopic map of a head, and defines the parallel-projecting mechanosensory pathways that elicit head grooming.
Figures






Similar articles
-
Somatotopic organization among parallel sensory pathways that promote a grooming sequence in Drosophila.Elife. 2024 Apr 18;12:RP87602. doi: 10.7554/eLife.87602. Elife. 2024. PMID: 38634460 Free PMC article.
-
A comprehensive mechanosensory connectome reveals a somatotopically organized neural circuit architecture controlling stimulus-aimed grooming of the Drosophila head.bioRxiv [Preprint]. 2025 Jun 25:2025.05.19.654894. doi: 10.1101/2025.05.19.654894. bioRxiv. 2025. PMID: 40501676 Free PMC article. Preprint.
-
Distinct subpopulations of mechanosensory chordotonal organ neurons elicit grooming of the fruit fly antennae.Elife. 2020 Oct 26;9:e59976. doi: 10.7554/eLife.59976. Elife. 2020. PMID: 33103999 Free PMC article.
-
Design of mechanosensory feedback during undulatory locomotion to enhance speed and stability.Curr Opin Neurobiol. 2023 Dec;83:102777. doi: 10.1016/j.conb.2023.102777. Epub 2023 Sep 2. Curr Opin Neurobiol. 2023. PMID: 37666012 Review.
-
Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance.Prog Brain Res. 1991;88:501-20. doi: 10.1016/s0079-6123(08)63830-3. Prog Brain Res. 1991. PMID: 1813931 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
