This is a preprint.
A highly efficient human cell-free translation system
- PMID: 36798401
- PMCID: PMC9934684
- DOI: 10.1101/2023.02.09.527910
A highly efficient human cell-free translation system
Update in
-
A highly efficient human cell-free translation system.RNA. 2023 Dec;29(12):1960-1972. doi: 10.1261/rna.079825.123. Epub 2023 Oct 4. RNA. 2023. PMID: 37793791 Free PMC article.
Abstract
Cell-free protein synthesis (CFPS) systems enable easy in vitro expression of proteins with many scientific, industrial, and therapeutic applications. Here we present an optimized, highly efficient human cell-free translation system that bypasses many limitations of currently used in vitro systems. This CFPS system is based on extracts from human HEK293T cells engineered to endogenously express GADD34 and K3L proteins, which suppress phosphorylation of translation initiation factor eIF2α. Overexpression of GADD34 and K3L proteins in human cells significantly simplifies cell lysate preparation. The new CFPS system improves the translation of 5' cap-dependent mRNAs as well as those that use internal ribosome entry site (IRES) mediated translation initiation. We find that expression of the GADD34 and K3L accessory proteins before cell lysis maintains low levels of phosphorylation of eIF2α in the extracts. During in vitro translation reactions, eIF2α phosphorylation increases moderately in a GCN2-dependent fashion that can be inhibited by GCN2 kinase inhibitors. We also find evidence for activation of regulatory pathways related to eukaryotic elongation factor 2 (eEF2) phosphorylation and ribosome quality control in the extracts. This new CFPS system should be useful for exploring human translation mechanisms in more physiological conditions outside the cell.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

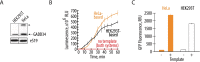


References
-
- Shimizu Y., Inoue A., Tomari Y., Suzuki T., Yokogawa T., Nishikawa K., Ueda T., Cell-free translation reconstituted with purified components. Nat. Biotechnol. 19, 751–755 (2001). - PubMed
-
- Spirin A. S., Baranov V. I., Ryabova L. A., Ovodov S. Y., Alakhov Y. B., A continuous cell-free translation system capable of producing polypeptides in high yield. Science. 242, 1162–1164 (1988). - PubMed
-
- Spirin A. S., High-throughput cell-free systems for synthesis of functionally active proteins. Trends Biotechnol. 22, 538–545 (2004). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
