This is a preprint.
Sensitivity of Rapid Antigen Tests Against SARS-CoV-2 Omicron and Delta Variants
- PMID: 36798414
- PMCID: PMC9934810
- DOI: 10.1101/2023.02.09.23285583
Sensitivity of Rapid Antigen Tests Against SARS-CoV-2 Omicron and Delta Variants
Update in
-
Sensitivity of rapid antigen tests against SARS-CoV-2 Omicron and Delta variants.J Clin Microbiol. 2023 Oct 24;61(10):e0013823. doi: 10.1128/jcm.00138-23. Epub 2023 Sep 20. J Clin Microbiol. 2023. PMID: 37728336 Free PMC article.
Abstract
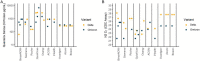
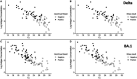
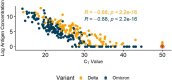
Rapid Antigen Tests (RAT) have become an invaluable tool for combating the COVID-19 pandemic. However, concerns have been raised regarding the ability of existing RATs to effectively detect emerging SARS-CoV-2 variants. We compared the performance of eight commercially available, emergency use authorized RATs against the Delta and Omicron SARS-CoV-2 variants using individual patient and serially diluted pooled clinical samples. The RATs exhibited lower sensitivity for Omicron samples when using PCR Cycle threshold (C T ) value (a proxy for RNA concentration) as the comparator. Interestingly, however, they exhibited similar sensitivity for Omicron and Delta samples when using quantitative antigen concentration as the comparator. We further found that the Omicron samples had lower ratios of antigen to RNA, which offers a potential explanation for the apparent lower sensitivity of RATs for that variant when using C T value as a reference. Our findings underscore the complexity in assessing RAT performance against emerging variants and highlight the need for ongoing evaluation in the face of changing population immunity and virus evolution.
Conflict of interest statement
Conflict-of-interest statement
ALG reports contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen, and Hologic, and research support from Gilead and Merck, outside of the described work.
The other authors have declared that no conflict of interest exists.
Figures
Similar articles
-
Sensitivity of rapid antigen tests against SARS-CoV-2 Omicron and Delta variants.J Clin Microbiol. 2023 Oct 24;61(10):e0013823. doi: 10.1128/jcm.00138-23. Epub 2023 Sep 20. J Clin Microbiol. 2023. PMID: 37728336 Free PMC article.
-
Analysis of seven SARS-CoV-2 rapid antigen tests in detecting omicron (B.1.1.529) versus delta (B.1.617.2) using cell culture supernatants and clinical specimens.Infection. 2023 Feb;51(1):239-245. doi: 10.1007/s15010-022-01844-5. Epub 2022 May 20. Infection. 2023. PMID: 35596057 Free PMC article.
-
Decreased Sensitivity of Rapid Antigen Test Is Associated with a Lower Viral Load of Omicron than Delta SARS-CoV-2 Variant.Microbiol Spectr. 2022 Oct 26;10(5):e0192222. doi: 10.1128/spectrum.01922-22. Epub 2022 Sep 20. Microbiol Spectr. 2022. PMID: 36125269 Free PMC article.
-
Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission.Rev Med Virol. 2022 Sep;32(5):e2381. doi: 10.1002/rmv.2381. Epub 2022 Jul 20. Rev Med Virol. 2022. PMID: 35856385 Free PMC article. Review.
-
Comparison of Omicron and Delta Variants of SARS-CoV-2: A Systematic Review of Current Evidence.Infect Disord Drug Targets. 2024;24(7):e050324227686. doi: 10.2174/0118715265279242240216114548. Infect Disord Drug Targets. 2024. PMID: 38445691
References
-
- Schrom J, Marquez C, Pilarowski G, Wang CY, Mitchell A, Puccinelli R, et al. Comparison of SARS-CoV-2 Reverse Transcriptase Polymerase Chain Reaction and BinaxNOW Rapid Antigen Tests at a Community Site During an Omicron Surge : A Cross-Sectional Study. Ann Intern Med. 2022;175(5):682–90. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
