This is a preprint.
Inverse-folding design of yeast telomerase RNA increases activity in vitro
- PMID: 36798419
- PMCID: PMC9934677
- DOI: 10.1101/2023.02.08.527468
Inverse-folding design of yeast telomerase RNA increases activity in vitro
Update in
-
Inverse-Folding Design of Yeast Telomerase RNA Increases Activity In Vitro.Noncoding RNA. 2023 Aug 28;9(5):51. doi: 10.3390/ncrna9050051. Noncoding RNA. 2023. PMID: 37736897 Free PMC article.
Abstract
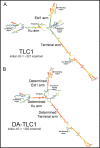
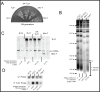
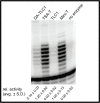
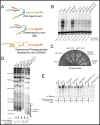
Saccharomyces cerevisiae telomerase RNA, TLC1, is an 1157 nt non-coding RNA that functions as both a template for DNA synthesis and a flexible scaffold for telomerase RNP holoenzyme protein subunits. The tractable budding yeast system has provided landmark discoveries about telomere biology in vivo , but yeast telomerase research has been hampered by the fact that the large TLC1 RNA subunit does not support robust telomerase activity in vitro . In contrast, 155-500 nt miniaturized TLC1 alleles comprising the catalytic core domain and lacking the RNA's long arms do reconstitute robust activity. We hypothesized that full-length TLC1 is prone to misfolding in vitro . To create a full-length yeast telomerase RNA predicted to fold into its biological relevant structure, we took an inverse RNA folding approach, changing 59 nucleotides predicted to increase the energetic favorability of folding into the modeled native structure based on the p-num feature of Mfold software. The sequence changes lowered the predicted ∆G in this "determined-arm" allele, DA-TLC1, by 61 kcal/mol (-19%) compared to wild type. We tested DA-TLC1 for reconstituted activity and found it to be ∼5-fold more robust than wild-type TLC1, suggesting that the inverse-folding design indeed improved folding in vitro into a catalytically active conformation. We also tested if DA-TLC1 functions in vivo and found that it complements a tlc1 ∆ strain, allowing cells to avoid senescence and maintain telomeres of nearly wild-type length. However, all inverse-designed RNAs that we tested had reduced abundance in vivo . In particular, inverse-designing nearly all of the Ku arm caused a profound reduction in telomerase RNA abundance in the cell and very short telomeres. Overall, these results show that inverse design of S. cerevisiae telomerase RNA increases activity in vitro , while reducing abundance in vivo . This study provides a biochemically and biologically tested approach to inverse-design RNAs using Mfold that could be useful for controlling RNA structure in basic research and biomedicine.
Figures
Similar articles
-
Inverse-Folding Design of Yeast Telomerase RNA Increases Activity In Vitro.Noncoding RNA. 2023 Aug 28;9(5):51. doi: 10.3390/ncrna9050051. Noncoding RNA. 2023. PMID: 37736897 Free PMC article.
-
Stiffened yeast telomerase RNA supports RNP function in vitro and in vivo.RNA. 2012 Sep;18(9):1666-78. doi: 10.1261/rna.033555.112. Epub 2012 Jul 31. RNA. 2012. PMID: 22850424 Free PMC article.
-
A miniature yeast telomerase RNA functions in vivo and reconstitutes activity in vitro.Nat Struct Mol Biol. 2005 Dec;12(12):1072-7. doi: 10.1038/nsmb1019. Epub 2005 Nov 20. Nat Struct Mol Biol. 2005. PMID: 16299517
-
Yeast Telomerase RNA Flexibly Scaffolds Protein Subunits: Results and Repercussions.Molecules. 2020 Jun 14;25(12):2750. doi: 10.3390/molecules25122750. Molecules. 2020. PMID: 32545864 Free PMC article. Review.
-
Telomerase: what are the Est proteins doing?Curr Opin Cell Biol. 2003 Jun;15(3):275-80. doi: 10.1016/s0955-0674(03)00040-1. Curr Opin Cell Biol. 2003. PMID: 12787768 Review.
References
-
- Watson J.D. Origin of concatemeric T7 DNA. Nat New Biol 239, 197–201 (1972). - PubMed
-
- Hayflick L. & Moorhead P.S. The serial cultivation of human diploid cell strains. Exp Cell Res 25, 585–621 (1961). - PubMed
-
- Weinert T.A. & Hartwell L.H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241, 317–22 (1988). - PubMed
-
- Greider C.W. & Blackburn E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–13 (1985). - PubMed
-
- Weinrich S.L. et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet 17, 498–502 (1997). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
