Pan‑cancer analysis of the deoxyribonuclease gene family
- PMID: 36798465
- PMCID: PMC9926046
- DOI: 10.3892/mco.2023.2615
Pan‑cancer analysis of the deoxyribonuclease gene family
Abstract
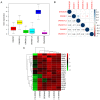
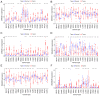
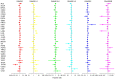
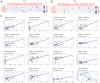
Deoxyribonuclease (DNase) is an enzyme that catalyzes the cleavage of phosphodiester bonds in the main chain of DNA to degrade DNA. DNase serves a vital role in several immune-related diseases. The present study linked the expression of DNase with overall survival (OS), performed pan-cancer co-expression analysis, and assessed the association between DNase and immune infiltration subtypes, tumor microenvironment and drug sensitivity through pan-cancer studies. Furthermore, gene expression data and clinical data were downloaded from The Cancer Genome Atlas. Next, through a series of bioinformatics analyses, DNase expression and survival, immune subtypes, tumor microenvironment and drug sensitivity in 33 tumor types were systematically studied. The expression of the DNase gene family was shown to have an apparent intratumoral heterogeneity. The expression of DNase 2, lysosomal (DNASE2) was the highest in tumors, whereas that of DNASE2 β was the lowest. DNase 1-like 3 (DNASE1L3) was mainly downregulated in tumors, whereas the rest of the DNases were mainly upregulated in tumors. The expression of DNase family members was also found to be associated with the OS rate of patients. DNase family genes may serve an essential role in the tumor microenvironment. DNase family gene expression was related to the content of cytotoxic cells, Immunescore, Stromalscore, Estimatescore and Tumorpurity. The present study also revealed that the DNase genes may be involved in the drug resistance of cancer cells. Finally, the correlation between DNase, and clinical stage and tumor microenvironment in hepatocellular carcinoma (HCC) was studied. In addition, the difference in DNASE1L3 expression between HCC and adjacent normal tissues, and the relationship between DNASE1L3 expression and clinical stage was verified by analyzing three groups in a Gene Expression Omnibus dataset and by performing immunohistochemistry. In conclusion, the present study assessed DNase gene expression, analyzed its relationship with patient OS, performed pan-cancer co-expression analysis, and assessed the association between DNase and immune infiltration subtypes, tumor microenvironment and drug sensitivity. The present study also confirmed the value of further laboratory research on DNases and their prospects in clinical cancer treatment.
Keywords: deoxyribonuclease; immune infiltration subtype; overall survival; tumor microenvironment; tumor stemness score.
Copyright: © Bai et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Integrative analysis of deoxyribonuclease 1-like 3 as a potential biomarker in renal cell carcinoma.Transl Androl Urol. 2023 Aug 31;12(8):1308-1320. doi: 10.21037/tau-23-355. Epub 2023 Aug 22. Transl Androl Urol. 2023. PMID: 37680233 Free PMC article.
-
A Pan-Cancer Analysis of the BIRC Gene Family and Its Association with Prognosis, Tumor Microenvironment, and Therapeutic Targets.Crit Rev Eukaryot Gene Expr. 2021;31(4):35-48. doi: 10.1615/CritRevEukaryotGeneExpr.2021038714. Crit Rev Eukaryot Gene Expr. 2021. PMID: 34587434
-
Pan-cancer analysis reveals interleukin-17 family members as biomarkers in the prediction for immune checkpoint inhibitor curative effect.Front Immunol. 2022 Sep 8;13:900273. doi: 10.3389/fimmu.2022.900273. eCollection 2022. Front Immunol. 2022. PMID: 36159856 Free PMC article.
-
A comprehensive analysis and validation of cuproptosis-associated genes across cancers: Overall survival, the tumor microenvironment, stemness scores, and drug sensitivity.Front Genet. 2022 Aug 29;13:939956. doi: 10.3389/fgene.2022.939956. eCollection 2022. Front Genet. 2022. PMID: 36105090 Free PMC article.
-
Cancer Stemness-Based Prognostic Immune-Related Gene Signatures in Lung Adenocarcinoma and Lung Squamous Cell Carcinoma.Front Endocrinol (Lausanne). 2021 Oct 21;12:755805. doi: 10.3389/fendo.2021.755805. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34745015 Free PMC article.
Cited by
-
Multimodal analysis of cell-free DNA enhances differentiation of early-stage breast cancer from benign lesions and healthy individuals.BMC Biol. 2025 Aug 20;23(1):259. doi: 10.1186/s12915-025-02371-z. BMC Biol. 2025. PMID: 40830871 Free PMC article.
-
Recent Advancements in Research on DNA Methylation and Testicular Germ Cell Tumors: Unveiling the Intricate Relationship.Biomedicines. 2024 May 8;12(5):1041. doi: 10.3390/biomedicines12051041. Biomedicines. 2024. PMID: 38791003 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
