pKr-2 induces neurodegeneration via upregulation of microglial TLR4 in the hippocampus of AD brain
- PMID: 36798617
- PMCID: PMC9926212
- DOI: 10.1016/j.bbih.2023.100593
pKr-2 induces neurodegeneration via upregulation of microglial TLR4 in the hippocampus of AD brain
Abstract
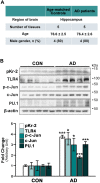
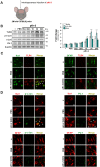
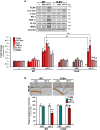
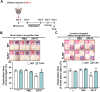
We recently demonstrated that prothrombin kringle-2 (pKr-2) derived from blood-brain barrier (BBB) disruption could induce hippocampal neurodegeneration and object recognition impairment through neurotoxic inflammatory responses in the five familial Alzheimer's disease mutation (5XFAD) mice. In the present study, we aimed to determine whether pKr-2 induces microglial activation by stimulating toll-like receptor 4 (TLR4) upregulation and examine whether this response contributes to pKr-2-induced neuroinflammatory damage in the hippocampi of mice models. We observed that inflammatory responses induced by pKr-2 administration in the hippocampi of wild-type mice were significantly abrogated in TLR4-deficient mice (TLR4-/-), and caffeine supply or rivaroxaban treatment that inhibits the overexpression of hippocampal pKr-2 reduced TLR4 upregulation in 5XFAD mice, resulting in the inhibition of neuroinflammatory responses. Similar to the expression patterns of pKr-2, TLR4, and the TLR4 transcription factors, PU.1 and p-c-Jun, seen in the postmortem hippocampal tissues of Alzheimer's disease (AD) patients, our results additionally showed the influence of transcriptional regulation on TLR4 expression following pKr-2 expression in triggering the production of neurotoxic inflammatory mediators. Therefore, we conclude that pKr-2 may play a role in initiating upregulation of microglial TLR4, consequently inducing hippocampal neurodegeneration. Furthermore, the control of pKr-2-induced microglial TLR4 could be a useful therapeutic strategy against hippocampal neurodegeneration in AD.
Keywords: Alzheimer's disease; Hippocampus; Microglia; Neurodegeneration; Neuroinflammation; Prothrombin kringle-2; Toll-like receptor 4.
© 2023 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Pathophysiological Role of Microglial Activation Induced by Blood-Borne Proteins in Alzheimer's Disease.Biomedicines. 2023 May 7;11(5):1383. doi: 10.3390/biomedicines11051383. Biomedicines. 2023. PMID: 37239054 Free PMC article. Review.
-
Control of hippocampal prothrombin kringle-2 (pKr-2) expression reduces neurotoxic symptoms in five familial Alzheimer's disease mice.Br J Pharmacol. 2022 Mar;179(5):998-1016. doi: 10.1111/bph.15681. Epub 2021 Oct 24. Br J Pharmacol. 2022. PMID: 34524687 Free PMC article.
-
Prothrombin Kringle-2: A Potential Inflammatory Pathogen in the Parkinsonian Dopaminergic System.Exp Neurobiol. 2016 Aug;25(4):147-55. doi: 10.5607/en.2016.25.4.147. Epub 2016 Aug 8. Exp Neurobiol. 2016. PMID: 27574481 Free PMC article. Review.
-
Induction of microglial toll-like receptor 4 by prothrombin kringle-2: a potential pathogenic mechanism in Parkinson's disease.Sci Rep. 2015 Oct 6;5:14764. doi: 10.1038/srep14764. Sci Rep. 2015. PMID: 26440368 Free PMC article.
-
Prothrombin kringle-2-induced oxidative stress contributes to the death of cortical neurons in vivo and in vitro: role of microglial NADPH oxidase.J Neuroimmunol. 2009 Sep 29;214(1-2):83-92. doi: 10.1016/j.jneuroim.2009.07.005. Epub 2009 Aug 5. J Neuroimmunol. 2009. PMID: 19660816
Cited by
-
The Crucial Role of the Blood-Brain Barrier in Neurodegenerative Diseases: Mechanisms of Disruption and Therapeutic Implications.J Clin Med. 2025 Jan 9;14(2):386. doi: 10.3390/jcm14020386. J Clin Med. 2025. PMID: 39860392 Free PMC article. Review.
-
Role of Oxidative Stress in Blood-Brain Barrier Disruption and Neurodegenerative Diseases.Antioxidants (Basel). 2024 Nov 28;13(12):1462. doi: 10.3390/antiox13121462. Antioxidants (Basel). 2024. PMID: 39765790 Free PMC article. Review.
-
Interplay between microglia and environmental risk factors in Alzheimer's disease.Neural Regen Res. 2024 Aug 1;19(8):1718-1727. doi: 10.4103/1673-5374.389745. Epub 2023 Dec 11. Neural Regen Res. 2024. PMID: 38103237 Free PMC article.
-
Pathophysiological Role of Microglial Activation Induced by Blood-Borne Proteins in Alzheimer's Disease.Biomedicines. 2023 May 7;11(5):1383. doi: 10.3390/biomedicines11051383. Biomedicines. 2023. PMID: 37239054 Free PMC article. Review.
References
-
- Arai T., Miklossy J., Klegeris A., Guo J.P., McGeer P.L. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 2006;65:19–25. - PubMed
-
- Behre G., Whitmarsh A.J., Coghlan M.P., Hoang T., Carpenter C.L., Zhang D.E., Davis R.J., Tenen D.G. c-Jun is a JNK-independent coactivator of the PU.1 transcription factor. J. Biol. Chem. 1999;274:4939–4946. - PubMed
-
- Bevins R.A., Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory. Nat. Protoc. 2006;1:1306–1311. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

