Rice DST transcription factor negatively regulates heat tolerance through ROS-mediated stomatal movement and heat-responsive gene expression
- PMID: 36798712
- PMCID: PMC9927019
- DOI: 10.3389/fpls.2023.1068296
Rice DST transcription factor negatively regulates heat tolerance through ROS-mediated stomatal movement and heat-responsive gene expression
Abstract
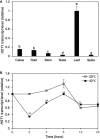
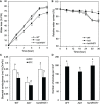
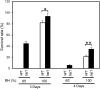
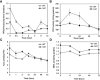
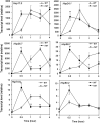
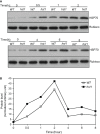
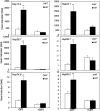
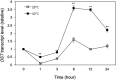
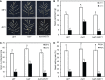
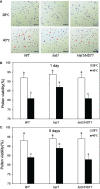
Plants are frequently subjected to a broad spectrum of abiotic stresses including drought, salinity and extreme temperatures and have evolved both common and stress-specific responses to promote fitness and survival. Understanding the components and mechanisms that underlie both common and stress-specific responses can enable development of crop plants tolerant to different stresses. Here, we report a rice heat stress-tolerant 1 (hst1) mutant with increased heat tolerance. HST1 encodes the DST transcription factor, which also regulates drought and salinity tolerance. Increased heat tolerance of hst1 was associated with suppressed expression of reactive oxygen species (ROS)-scavenging peroxidases and increased ROS levels, which reduced water loss by decreasing stomatal aperture under heat stress. In addition, increased ROS levels enhanced expression of genes encoding heat shock protein (HSPs) including HSP80, HSP74, HSP58 and small HSPs. HSPs promote stabilization of proteins and protein refolding under heat stress and accordingly mutation of HST1 also improved reproductive traits including pollen viability and seed setting under high temperature. These results broaden the negative roles of DST in abiotic stress tolerance and provide important new insights into DST-regulated tolerance to diverse abiotic stresses through both shared and stress-specific mechanisms.
Keywords: DST transcription factor; heat shock genes; plant heat tolerance; reactive oxygen species; reproductive traits under heat stress; stomatal aperture.
Copyright © 2023 Ding, Zhou, Wang, Qu, Hu, Jiang, Yi, Wang, Cai, Zhu and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Rice histone deacetylase HDA704 positively regulates drought and salt tolerance by controlling stomatal aperture and density.Planta. 2021 Sep 20;254(4):79. doi: 10.1007/s00425-021-03729-7. Planta. 2021. PMID: 34542712
-
A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control.Genes Dev. 2009 Aug 1;23(15):1805-17. doi: 10.1101/gad.1812409. Genes Dev. 2009. PMID: 19651988 Free PMC article.
-
DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice.PLoS Genet. 2015 Oct 23;11(10):e1005617. doi: 10.1371/journal.pgen.1005617. eCollection 2015 Oct. PLoS Genet. 2015. PMID: 26496194 Free PMC article.
-
Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: key regulators and possible mechanisms.Protoplasma. 2018 Jan;255(1):399-412. doi: 10.1007/s00709-017-1150-8. Epub 2017 Aug 4. Protoplasma. 2018. PMID: 28776104 Review.
-
Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses.Physiol Plant. 2021 Jul;172(3):1711-1723. doi: 10.1111/ppl.13373. Epub 2021 Mar 1. Physiol Plant. 2021. PMID: 33605458 Review.
Cited by
-
Artificial miRNAs and target-mimics as potential tools for crop improvement.Physiol Mol Biol Plants. 2025 Jan;31(1):67-91. doi: 10.1007/s12298-025-01550-0. Epub 2025 Jan 17. Physiol Mol Biol Plants. 2025. PMID: 39901962 Review.
-
Overexpression of wheat C2H2 zinc finger protein transcription factor TaZAT8-5B enhances drought tolerance and root growth in Arabidopsis thaliana.Planta. 2024 Oct 28;260(6):126. doi: 10.1007/s00425-024-04559-z. Planta. 2024. PMID: 39466433
-
How Rice Responds to Temperature Changes and Defeats Heat Stress.Rice (N Y). 2024 Nov 29;17(1):73. doi: 10.1186/s12284-024-00748-2. Rice (N Y). 2024. PMID: 39611857 Free PMC article. Review.
-
Calcium Signaling and the Response to Heat Shock in Crop Plants.Int J Mol Sci. 2023 Dec 26;25(1):324. doi: 10.3390/ijms25010324. Int J Mol Sci. 2023. PMID: 38203495 Free PMC article. Review.
-
Stomata-Photosynthesis Synergy Mediates Combined Heat and Salt Stress Tolerance in Sugarcane Mutant M4209.Plant Cell Environ. 2025 Jun;48(6):4668-4684. doi: 10.1111/pce.15424. Epub 2025 Mar 7. Plant Cell Environ. 2025. PMID: 40052246 Free PMC article.
References
LinkOut - more resources
Full Text Sources

