Clinical performance evaluation of serum amyloid A module of Mindray BC-7500CS automated hematology analyzer
- PMID: 36798927
- PMCID: PMC9926133
- DOI: 10.21037/tp-22-661
Clinical performance evaluation of serum amyloid A module of Mindray BC-7500CS automated hematology analyzer
Abstract
Background: Laboratory detection of high values of serum amyloid A (SAA) is impaired by the hook effect. In response to this problem, Mindray has launched the new generation BC-7500CS automated hematology analyzer with an SAA autodilution (SAA-D) function. The present study aimed to verify the performance of the SAA module.
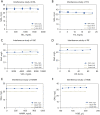
Methods: Venous whole-blood specimens anticoagulated with EDTA-K2 were randomly collected from outpatients and inpatient of the Children's Hospital of Nanjing Medical University (CH). Background, repeatability, precision, linear range, intermode comparison, and interference of the SAA module of the Mindray BC-7500CS were evaluated, and the performance of the SAA-D function was verified.
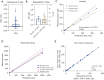
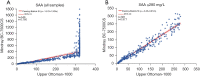
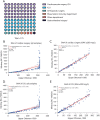
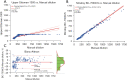
Results: The Mindray BC-7500CS showed an SAA background of 0.14 mg/L, well below that claimed by the manufacturer. Repeatability of SAA with standard deviation (SD) <0.6 mg/L and coefficient of variation (CV) <6%, the quality control (QC) precision was less than 8%. The measured value of the linear range was essentially consistent with the theoretical value, and the maximum measured values could reach 1932.38 mg/L. The deviation between whole-blood mode and micro-whole-blood mode was small (r=0.999), and the SAA module displayed high anti-interference ability. In addition, the measured results of specimens with high SAA concentration diluted by SAA-D were close to those after manual dilution (r=0.993).
Conclusions: The SAA module of the Mindray BC-7500CS had excellent performance, and the SAA-D function was highly accurate at measuring specimens with high SAA concentration, enabling reliable SAA detection in the laboratory and clinical practice.
Keywords: BC-7500CS; SAA autodilution (SAA-D); high SAA concentration; performance evaluation; serum amyloid A (SAA).
2023 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-661/coif). The authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources
