Acute effects of moderate vs. vigorous endurance exercise on urinary metabolites in healthy, young, physically active men-A multi-platform metabolomics approach
- PMID: 36798943
- PMCID: PMC9927024
- DOI: 10.3389/fphys.2023.1028643
Acute effects of moderate vs. vigorous endurance exercise on urinary metabolites in healthy, young, physically active men-A multi-platform metabolomics approach
Abstract
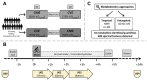
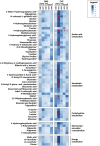
Introduction: Endurance exercise alters whole-body as well as skeletal muscle metabolism and physiology, leading to improvements in performance and health. However, biological mechanisms underlying the body's adaptations to different endurance exercise protocols are not entirely understood. Methods: We applied a multi-platform metabolomics approach to identify urinary metabolites and associated metabolic pathways that distinguish the acute metabolic response to two endurance exercise interventions at distinct intensities. In our randomized crossover study, 16 healthy, young, and physically active men performed 30 min of continuous moderate exercise (CME) and continuous vigorous exercise (CVE). Urine was collected during three post-exercise sampling phases (U01/U02/U03: until 45/105/195 min post-exercise), providing detailed temporal information on the response of the urinary metabolome to CME and CVE. Also, fasting spot urine samples were collected pre-exercise (U00) and on the following day (U04). While untargeted two-dimensional gas chromatography-mass spectrometry (GC×GC-MS) led to the detection of 608 spectral features, 44 metabolites were identified and quantified by targeted nuclear magnetic resonance (NMR) spectroscopy or liquid chromatography-mass spectrometry (LC-MS). Results: 104 urinary metabolites showed at least one significant difference for selected comparisons of sampling time points within or between exercise trials as well as a relevant median fold change >1.5 or <0. (NMR, LC-MS) or >2.0 or <0.5 (GC×GC-MS), being classified as either exercise-responsive or intensity-dependent. Our findings indicate that CVE induced more profound alterations in the urinary metabolome than CME, especially at U01, returning to baseline within 24 h after U00. Most differences between exercise trials are likely to reflect higher energy requirements during CVE, as demonstrated by greater shifts in metabolites related to glycolysis (e.g., lactate, pyruvate), tricarboxylic acid cycle (e.g., cis-aconitate, malate), purine nucleotide breakdown (e.g., hypoxanthine), and amino acid mobilization (e.g., alanine) or degradation (e.g., 4-hydroxyphenylacetate). Discussion: To conclude, this study provided first evidence of specific urinary metabolites as potential metabolic markers of endurance exercise intensity. Future studies are needed to validate our results and to examine whether acute metabolite changes in urine might also be partly reflective of mechanisms underlying the health- or performance-enhancing effects of endurance exercise, particularly if performed at high intensities.
Keywords: GC×GC-MS; LC-MS; NMR; continuous physical exercise; exercise intensity; exercise metabolomics; metabolic markers; urine metabolome.
Copyright © 2023 Kistner, Mack, Rist, Krüger, Egert, Biniaminov, Engelbert, Seifert, Dörr, Ferrario, Neumann, Altmann and Bub.
Conflict of interest statement
Author SA was employed by the company TSG ResearchLab gGmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
High-Intensity Interval Training Decreases Resting Urinary Hypoxanthine Concentration in Young Active Men-A Metabolomic Approach.Metabolites. 2019 Jul 10;9(7):137. doi: 10.3390/metabo9070137. Metabolites. 2019. PMID: 31295919 Free PMC article.
-
An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans-Results of the KarMeN Study.Metabolites. 2020 May 21;10(5):212. doi: 10.3390/metabo10050212. Metabolites. 2020. PMID: 32455749 Free PMC article.
-
The combination of four analytical methods to explore skeletal muscle metabolomics: Better coverage of metabolic pathways or a marketing argument?J Pharm Biomed Anal. 2018 Jan 30;148:273-279. doi: 10.1016/j.jpba.2017.10.013. Epub 2017 Oct 18. J Pharm Biomed Anal. 2018. PMID: 29059617
-
Mass spectrometric based approaches in urine metabolomics and biomarker discovery.Mass Spectrom Rev. 2017 Mar;36(2):115-134. doi: 10.1002/mas.21455. Epub 2015 Apr 16. Mass Spectrom Rev. 2017. PMID: 25881008 Review.
-
Exercise Metabolome: Insights for Health and Performance.Metabolites. 2023 May 26;13(6):694. doi: 10.3390/metabo13060694. Metabolites. 2023. PMID: 37367852 Free PMC article. Review.
Cited by
-
Acute changes in urinary metabolites: vinyasa yoga compared to cycle ergometer exercise.Front Sports Act Living. 2025 Apr 16;7:1556989. doi: 10.3389/fspor.2025.1556989. eCollection 2025. Front Sports Act Living. 2025. PMID: 40309030 Free PMC article.
-
Exercise intensity determines circulating levels of Lac-Phe and other exerkines: a randomized crossover trial.Metabolomics. 2025 May 7;21(3):63. doi: 10.1007/s11306-025-02260-0. Metabolomics. 2025. PMID: 40335829 Free PMC article. Clinical Trial.
References
-
- Armbruster M., Rist M., Seifert S., Frommherz L., Weinert C., Mack C., et al. (2018). Metabolite profiles evaluated, according to sex, do not predict resting energy expenditure and lean body mass in healthy non-obese subjects. Eur. J. Nutr. 58, 2207–2217. 10.1007/s00394-018-1767-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

