Is local review of positron emission tomography scans sufficient in diffuse large B-cell lymphoma clinical trials? A CALGB 50303 analysis
- PMID: 36799072
- PMCID: PMC10134372
- DOI: 10.1002/cam4.5628
Is local review of positron emission tomography scans sufficient in diffuse large B-cell lymphoma clinical trials? A CALGB 50303 analysis
Abstract
Background: Quantitative methods of Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) interpretation, including the percent change in FDG uptake from baseline (ΔSUV), are under investigation in lymphoma to overcome challenges associated with visual scoring systems (VSS) such as the Deauville 5-point scale (5-PS).
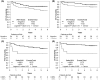
Methods: In CALGB 50303, patients with DLBCL received frontline R-CHOP or DA-EPOCH-R, and although there were no significant associations between interim PET responses assessed centrally after cycle 2 (iPET) using 5-PS with progression-free survival (PFS) or overall survival (OS), there were significant associations between central determinations of iPET ∆SUV with PFS/OS. In this patient cohort, we retrospectively compared local vs central iPET readings and evaluated associations between local imaging data and survival outcomes.
Results: Agreement between local and central review was moderate (kappa = 0.53) for VSS and high (kappa = 0.81) for ∆SUV categories (<66% vs. ≥66%). ∆SUV ≥66% at iPET was significantly associated with PFS (p = 0.03) and OS (p = 0.002), but VSS was not. Associations with PFS/OS when applying local review vs central review were comparable.
Conclusions: These data suggest that local PET interpretation for response determination may be acceptable in clinical trials. Our findings also highlight limitations of VSS and call for incorporation of more objective measures of response assessment in clinical trials.
Keywords: Deauville 5-PS; International Harmonization Project criteria; interim PET; visual scoring system; ΔSUV.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Figures
References
-
- Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging Subcommittee of International Harmonization Project in lymphoma. J Clin Oncol. 2007;25(5):571‐578. - PubMed
-
- Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the first international workshop on interim‐PET scan in lymphoma. Leuk Lymphoma. 2009;50(8):1257‐1260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous