Long-Term Efficacy of Erythropoiesis-Stimulating Agents in Patients with Low-Risk or Intermediate-1-Risk Myelodysplastic Syndrome: Multicenter Real-Life Data
- PMID: 36799095
- PMCID: PMC10240155
- DOI: 10.4274/tjh.galenos.2023.2022.0437
Long-Term Efficacy of Erythropoiesis-Stimulating Agents in Patients with Low-Risk or Intermediate-1-Risk Myelodysplastic Syndrome: Multicenter Real-Life Data
Abstract
Objective: This study was undertaken to evaluate the long-term clinical efficacy of epoetin alfa and darbepoetin alfa in patients with myelodysplastic syndrome (MDS) in a real-life setting.
Materials and methods: A total of 204 patients with low-risk or intermediate-1-risk MDS who received epoetin alfa or darbepoetin alfa were included. Hemoglobin levels and transfusion needs were recorded before treatment and at 12 months, 24 months, 36 months, and 48 months of treatment.
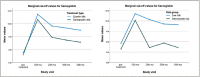
Results: At the 36-month (p=0.025) and 48-month (p=0.022) visits, epoetin alfa yielded significantly higher hemoglobin levels compared to darbepoetin alfa. Transfusion needs were also significantly lower with epoetin alfa compared to darbepoetin alfa at 24 months (p=0.012) and in the low-risk group compared to the intermediate-risk group at 24 months (p=0.018), 36 months (p=0.025), and 48 months (p<0.001). Treatment response rates at the 24-month, 36-month, and 48-month visits in the epoetin alfa (43.0%, 33.6%, and 27.1%), darbepoetin alfa (29.9%, 22.7%, and 16.5%), low-risk (39.3%, 30.0%, and 26.0%), and intermediate-risk (29.6%, 24.1%, and 11.1%) groups were lower than those obtained at 12 months, and the values differed significantly for the 36-month and 48-month visits with values ranging from p<0.05 to p<0.001.
Conclusion: This real-life long-term ESA extension study investigated the clinical efficacy of epoetin alfa and darbepoetin alfa for up to 48 months, revealing that treatment efficacy reached a plateau starting from the 24th month of therapy with a continuing decrease in treatment response rates regardless of treatment type, risk status, or gender. Nonetheless, significantly higher hemoglobin levels and marked improvement in transfusion needs were evident in epoetin-treated patients compared to darbepoetin-treated patients and in the low-risk group compared to the intermediate-risk group.
Amaç: Myelodisplastik sendromlu (MDS) hastalarda epoetin alfa ve darbepoetin alfa tedavisinin gerçek yaşam ortamında uzun-dönem klinik etkinliğini değerlendirmek.
Gereç ve yöntemler: Bu çalışmaya düşük veya orta-1 risk grubu MDS tanısı ile epoetin alfa veya darbepoetin alfa tedavisi almış 204 hasta dahil edildi. Hemoglobin düzeyleri ve transfüzyon gereksinimi, tedaviden önce ve tedavinin 12., 24., 36. ve 48. aylarında değerlendirildi.
Bulgular: Epoetin alfa, darbepoetin alfa ile kıyaslandığında, 36. ay (p=0,025) ve 48. aylarda (p=0,022) anlamlı şekilde daha yüksek hemoglobin düzeylerini sağladı. Transfüzyon gereksinimi 24. ayda (p=0,012) epoetin alfa grubunda darbepoetin alfa grubuna göre, 24. ay (p=0,018), 36. ay (p=0,025) ve 48. aylarda (p<0,001) ise düşük risk grubunda orta risk grubuna göre anlamlı şekilde daha düşük olarak bulundu. Tedavi yanıt oranları 24. ay, 36. ay ve 48. aylarda epoetin alfa (%43,0, %33,6 ve %27,1), darbepoetin alfa (%29,9, %22,7 ve %16,5), düşük risk (%39,3, %30,0 ve %26,0) ve orta risk (%29,6, %24,1 ve %11,1) gruplarında 12. ay yanıt oranlarına göre daha düşük olup, 36. ve 48. aylarda bu değişim istatistiksel olarak anlamlı idi (p<0,05 ile <0,001 arası).
Sonuç: Epoetin alfa ve darbepoetin alfanın 48 aylık klinik etkililiğinin değerlendirildiği bu gerçek-yaşam uzun-dönem ESA çalışmasında, tedavi etkililiğinin tedavinin 24. ayından başlayarak plato evresine eriştiği ve devamında tedavi yanıt oranlarında, tedavi tipi, risk durumu veya cinsiyetten bağımsız olarak süregiden bir düşüşün gerçekleştiği saptandı. Bununla birlikte, epoetin tedavisi alan grupta darbepoetin ile tedavi edilen gruba göre ve düşük risk grubu hastalarda orta risk grubu hastalara göre, hemoglobin düzeyleri anlamlı şekilde daha yüksek olup, transfüzyon gereksiniminde de belirgin azalma olduğu tespit edildi.
Keywords: Myelodysplastic syndrome; Low-risk; Intermediate-1-risk; Epoetin alfa; Darbepoetin alfa; Long-term; Treatment response; Duration of response; Transfusion dependence.
©Copyright 2023 by Turkish Society of Hematology | Turkish Journal of Hematology, Published by Galenos Publishing House
Conflict of interest statement
Conflict of Interest: No conflict of interest was declared by the authors.
Figures
References
-
- Park S, Greenberg P, Yucel A, Farmer C, O’Neill F, De Oliveira Brandao C, Fenaux P. Clinical effectiveness and safety of erythropoietin-stimulating agents for the treatment of low- and intermediate-1-risk myelodysplastic syndrome: a systematic literature review. Br J Haematol. 2019;184:134–160. - PubMed
-
- Malcovati L, Hellström-Lindberg E, Bowen D, Adès L, Cermak J, Del Cañizo C, Della Porta MG, Fenaux P, Gattermann N, Germing U, Jansen JH, Mittelman M, Mufti G, Platzbecker U, Sanz GF, Selleslag D, Skov-Holm M, Stauder R, Symeonidis A, van de Loosdrecht AA, de Witte T, Cazzola M; European LeukemiaNet. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122:2943–2964. - PMC - PubMed
-
- Fenaux P, Haase D, Sanz GF, Santini V, Buske C; ESMO Guidelines Working Group. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):57–69. - PubMed
-
- Killick SB, Carter C, Culligan D, Dalley C, Das-Gupta E, Drummond M, Enright H, Jones GL, Kell J, Mills J, Mufti G, Parker J, Raj K, Sternberg A, Vyas P, Bowen D; British Committee for Standards in Haematology. Guidelines for the diagnosis and management of adult myelodysplastic syndromes. Br J Haematol. 2014;164:503–525. - PubMed
-
- Park S, Kelaidi C, Sapena R, Vassilieff D, Beyne-Rauzy O, Coiteux V, Vey N, Ravoet C, Cheze S, Rose C, Legros L, Stamatoullas A, Escoffre-Barbe M, Guerci A, Chaury MP, Fenaux P, Dreyfus F. Early introduction of ESA in low risk MDS patients may delay the need for RBC transfusion: a retrospective analysis on 112 patients. Leuk Res. 2010;34:1430–1436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous