Zinc finger protein‑like 1 is a novel neuroendocrine biomarker for prostate cancer
- PMID: 36799165
- PMCID: PMC9937688
- DOI: 10.3892/ijo.2023.5486
Zinc finger protein‑like 1 is a novel neuroendocrine biomarker for prostate cancer
Retraction in
-
[Retracted] Zinc finger protein‑like 1 is a novel neuroendocrine biomarker for prostate cancer.Int J Oncol. 2025 Mar;66(3):16. doi: 10.3892/ijo.2025.5722. Epub 2025 Feb 7. Int J Oncol. 2025. PMID: 39917995 Free PMC article.
Abstract
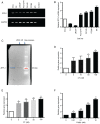
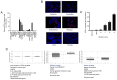
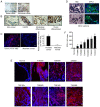
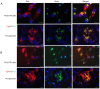
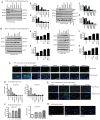
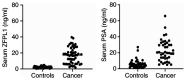
Prostate‑derived calcitonin (CT) and its receptor induce tumorigenicity and increase metastatic potential of prostate cancer (PC). CT‑inducible genes in human prostate were identified by subtraction hybridization. Among these genes, zinc finger protein like 1 (ZFPL1) protein was interesting since it was abundantly expressed in malignant prostates but was almost absent in benign prostates. ZFPL1 expression was upregulated by CT and androgens, and ZFPL1 protein was secreted by prostate tumor cells through exosomal secretion. Serum levels of ZFPL1 in cancer patients were at least 4‑fold higher than those in the sera of cancer‑free individuals. Cell biology of ZFPL1 suggests its localization in Golgi bodies and exosomes, and its colocalization with chromogranin A and CD44. These results suggested that ZFPL1 is secreted by tumor cells of neuroendocrine (NE)/stem cell phenotype. The knockdown of endogenous ZFPL1 in (PC) cells led to a remarkable decrease in cell proliferation, and invasion while increasing their apoptosis. As expected, the overexpression of ZFPL1 in prostate cells had an opposite effect on these functions. The knockdown of ZFPL1 in PC cells also decreased Akt phosphorylation, suggesting the actions of ZFPL1 may be mediated through the PI3K‑Akt pathway. Moreover, the present results revealed that ZFPL1 is released by tumors cells of NE or androgen‑independent phenotype and its serum levels are significantly higher in cancer patients, suggesting that it may serve as a blood‑based non‑invasive biomarker of aggressive PC.
Keywords: calcitonin; cancer marker; neuroendocrine; prostate cancer; zinc finger protein like 1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Giona S. The Epidemiology of Prostate Cancer. Prostate Cancer Exon Publications; Australia: 2021. pp. 1–16. - PubMed
-
- Schroder FH, Hugosson J, Carlsson S, Tammela T, Määttänen L, Auvinen A, Kwiatkowski M, Recker F, Roobol MJ. Screening for prostate cancer decreases the risk of developing metastatic disease: Findings from the European Randomized study of screening for prostate cancer (ERSPC) Eur Urol. 2012;62:745–752. doi: 10.1016/j.eururo.2012.05.068. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
