Limosilactobacillus reuteri normalizes blood-brain barrier dysfunction and neurodevelopment deficits associated with prenatal exposure to lipopolysaccharide
- PMID: 36799469
- PMCID: PMC9980478
- DOI: 10.1080/19490976.2023.2178800
Limosilactobacillus reuteri normalizes blood-brain barrier dysfunction and neurodevelopment deficits associated with prenatal exposure to lipopolysaccharide
Abstract
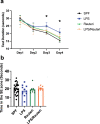
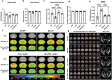
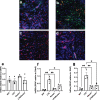
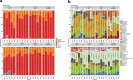
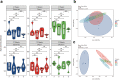
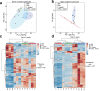
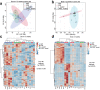
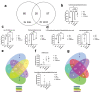
Maternal immune activation (MIA) derived from late gestational infection such as seen in chorioamnionitis poses a significantly increased risk for neurodevelopmental deficits in the offspring. Manipulating early microbiota through maternal probiotic supplementation has been shown to be an effective means to improve outcomes; however, the mechanisms remain unclear. In this study, we demonstrated that MIA modeled by exposing pregnant dams to lipopolysaccharide (LPS) induced an underdevelopment of the blood vessels, an increase in permeability and astrogliosis of the blood-brain barrier (BBB) at prewean age. The BBB developmental and functional deficits early in life impaired spatial learning later in life. Maternal Limosilactobacillus reuteri (L. reuteri) supplementation starting at birth rescued the BBB underdevelopment and dysfunction-associated cognitive function. Maternal L. reuteri-mediated alterations in β-diversity of the microbial community and metabolic responses in the offspring provide mechanisms and potential targets for promoting BBB integrity and long-term neurodevelopmental outcomes.
Keywords: Maternal inflammation; blood–brain barrier; lipopolysaccharide; probiotics.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Han VX, Patel S, Jones HF, Nielsen TC, Mohammad SS, Hofer MJ, Gold W, Brilot F, Lain SJ, Nassar N, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry. 2021;11(71). doi:10.1038/s41398-021-01198-w - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
