From Immunogen to COVID-19 vaccines: Prospects for the post-pandemic era
- PMID: 36800265
- PMCID: PMC9805901
- DOI: 10.1016/j.biopha.2022.114208
From Immunogen to COVID-19 vaccines: Prospects for the post-pandemic era
Abstract
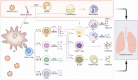
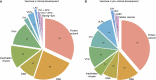
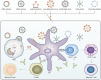
The COVID-19 pandemic has affected millions of people and posed an unprecedented burden on healthcare systems and economies worldwide since the outbreak of the COVID-19. A considerable number of nations have investigated COVID-19 and proposed a series of prevention and treatment strategies thus far. The pandemic prevention strategies implemented in China have suggested that the spread of COVID-19 can be effectively reduced by restricting large-scale gathering, developing community-scale nucleic acid testing, and conducting epidemiological investigations, whereas sporadic cases have always been identified in numerous places. Currently, there is still no decisive therapy for COVID-19 or related complications. The development of COVID-19 vaccines has raised the hope for mitigating this pandemic based on the intercross immunity induced by COVID-19. Thus far, several types of COVID-19 vaccines have been developed and released to into financial markets. From the perspective of vaccine use in globe, COVID-19 vaccines are beneficial to mitigate the pandemic, whereas the relative adverse events have been reported progressively. This is a review about the development, challenges and prospects of COVID-19 vaccines, and it can provide more insights into all aspects of the vaccines.
Keywords: Adverse reactions; COVID-19; Complications; Contraindications; Vaccine.
Copyright © 2023 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Conflict of interest statement No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

