SGN-CD228A Is an Investigational CD228-Directed Antibody-Drug Conjugate with Potent Antitumor Activity across a Wide Spectrum of Preclinical Solid Tumor Models
- PMID: 36800443
- PMCID: PMC10068445
- DOI: 10.1158/1535-7163.MCT-22-0401
SGN-CD228A Is an Investigational CD228-Directed Antibody-Drug Conjugate with Potent Antitumor Activity across a Wide Spectrum of Preclinical Solid Tumor Models
Abstract
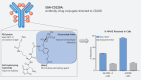
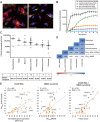
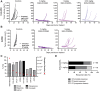
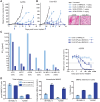
SGN-CD228A is an investigational antibody-drug conjugate (ADC) directed to melanotransferrin (CD228, MELTF, MFI2, p97), a cell-surface protein first identified in melanoma. SGN-CD228A consists of a humanized antibody, hL49, with high specificity and affinity for CD228 that is stably conjugated to 8 molecules of the clinically validated microtubule-disrupting agent monomethyl auristatin E (MMAE) via a novel glucuronide linker. We performed comprehensive IHC studies, which corroborated published RNA sequencing data and confirmed low CD228 expression in normal tissues and high expression in several cancers, including melanoma, squamous non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), colorectal cancer, and pancreatic cancer. SGN-CD228A was efficiently internalized in various tumor cell types, and its cytotoxic activity was dependent on CD228 expression and internalization and intrinsic sensitivity to the MMAE payload. Compared with the valine-citrulline dipeptide linker, the novel glucuronide linker increased the cellular retention of MMAE in vitro and conferred improved antitumor activity against melanoma cell lines in vitro and in vivo. In addition, SGN-CD228A was active across melanoma, TNBC, and NSCLC cell line- and patient-derived xenograft models with heterogeneous antigen expression. In vivo, CD228 expression was important for response to SGN-CD228A but was not well correlated across all tumor types, suggesting that other factors associated with ADC activity are important. Overall, SGN-CD228A is a CD228-directed, investigational ADC that employs innovative technology and has compelling preclinical antitumor activity. SGN-CD228A is investigated in a Phase I clinical trial (NCT04042480) in patients with advanced solid tumors.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
- 1535-7163. doi: 10.1158/1535-7163.MCT-22-4-HI doi: 10.1158/1535-7163.MCT-22-4-HI
References
-
- Brown JP, Hewick RM, Hellstrom I, Hellstrom KE, Doolittle RF, Dreyer WJ. Human melanoma-associated antigen p97 is structurally and functionally related to transferrin. Nature 1982;296:171–3. - PubMed
-
- Demeule M, Poirier J, Jodoin J, Bertrand Y, Desrosiers RR, Dagenais C, et al. High transcytosis of melanotransferrin (P97) across the blood-brain barrier. J Neurochem 2002;83:924–33. - PubMed
-
- Dus-Szachniewicz K, Ostasiewicz P, Wozniak M, Kolodziej P, Wisniewski JR, Ziolkowski P. Pattern of melanotransferrin expression in human colorectal tissues: an immunohistochemical study on potential clinical application. Anticancer Res 2015;35:6551–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

