Podocyte-Parietal Epithelial Cell Interdependence in Glomerular Development and Disease
- PMID: 36800545
- PMCID: PMC10125654
- DOI: 10.1681/ASN.0000000000000104
Podocyte-Parietal Epithelial Cell Interdependence in Glomerular Development and Disease
Abstract
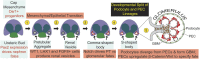
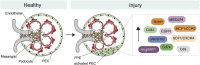
Podocytes and parietal epithelial cells (PECs) are among the few principal cell types within the kidney glomerulus, the former serving as a crucial constituent of the kidney filtration barrier and the latter representing a supporting epithelial layer that adorns the inner wall of Bowman's capsule. Podocytes and PECs share a circumscript developmental lineage that only begins to diverge during the S-shaped body stage of nephron formation-occurring immediately before the emergence of the fully mature nephron. These two cell types, therefore, share a highly conserved gene expression program, evidenced by recently discovered intermediate cell types occupying a distinct spatiotemporal gene expression zone between podocytes and PECs. In addition to their homeostatic functions, podocytes and PECs also have roles in kidney pathogenesis. Rapid podocyte loss in diseases, such as rapidly progressive GN and collapsing and cellular subtypes of FSGS, is closely allied with PEC proliferation and migration toward the capillary tuft, resulting in the formation of crescents and pseudocrescents. PECs are thought to contribute to disease progression and severity, and the interdependence between these two cell types during development and in various manifestations of kidney pathology is the primary focus of this review.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
Y. Gowthaman reports Research Funding: NIH/NIDDK and Veterans Affairs. S.K. Mallipattu reports Consultancy: L.E.K. Consulting and Wildwood Therapeutics, Inc.; Research Funding: Dialysis Clinic Inc. and Spectral Medical Inc.; Patents: Krüppel-like factor 15 (KLF15) Small Molecule Agonists in Kidney Disease, US 63/018.247, April 30, 2021; and Advisory or Leadership Role: Clinically Integrated Network Board Member (Accountable Care Organization, LLC Stony Brook Medicine). D.J. Salant reports Consultancy: Advance Medical, Pfizer, UpToDate, and Visterra; Research Funding: NIH; Honoraria: Several academic institutions and national societies; Patents or Royalties: Patent: “Diagnostics in membranous nephropathy”–Boston Medical Center; Advisory or Leadership Role: Editorial board:
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

