Incidence, risk factors and pre-emptive screening for COVID-19 associated pulmonary aspergillosis in an era of immunomodulant therapy
- PMID: 36801598
- PMCID: PMC9934852
- DOI: 10.1016/j.jcrc.2023.154272
Incidence, risk factors and pre-emptive screening for COVID-19 associated pulmonary aspergillosis in an era of immunomodulant therapy
Abstract
Purpose: COVID-19 associated pulmonary aspergillosis (CAPA) is associated with increased morbidity and mortality in ICU patients. We investigated the incidence of, risk factors for and potential benefit of a pre-emptive screening strategy for CAPA in ICUs in the Netherlands/Belgium during immunosuppressive COVID-19 treatment.
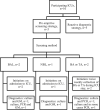
Materials and methods: A retrospective, observational, multicentre study was performed from September 2020-April 2021 including patients admitted to the ICU who had undergone diagnostics for CAPA. Patients were classified based on 2020 ECMM/ISHAM consensus criteria.
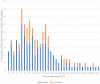
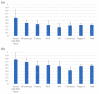
Results: CAPA was diagnosed in 295/1977 (14.9%) patients. Corticosteroids were administered to 97.1% of patients and interleukin-6 inhibitors (anti-IL-6) to 23.5%. EORTC/MSGERC host factors or treatment with anti-IL-6 with or without corticosteroids were not risk factors for CAPA. Ninety-day mortality was 65.3% (145/222) in patients with CAPA compared to 53.7% (176/328) without CAPA (p = 0.008). Median time from ICU admission to CAPA diagnosis was 12 days. Pre-emptive screening for CAPA was not associated with earlier diagnosis or reduced mortality compared to a reactive diagnostic strategy.
Conclusions: CAPA is an indicator of a protracted course of a COVID-19 infection. No benefit of pre-emptive screening was observed, but prospective studies comparing pre-defined strategies would be required to confirm this observation.
Keywords: Aspergillus fumigatus; CAPA; COVID-19; Intensive care unit; Invasive fungal infections; Invasive pulmonary aspergillosis; SARS-CoV-2.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Paul Verweij: Institution contracted for research grant: F2G and Gilead Sciences; Honoraria for lectures paid to institution: F2G, Gilead Sciences, Pfizer; Participation on a Data Safety Monitoring Board or Advisory Board paid to institution: F2G, Mundipharma. Roger Brüggeman: Institution contracted for research grant: Astellas Pharma, Inc., Gilead Sciences, Merck Sharp & Dohme Corp., Pfizer; Consulting fees paid to institution: Astellas Pharma, Inc., F2G, Amplyx, Gilead Sciences, Merck Sharp & Dohme Corp., Mundipharma, Pfizer. Joost Wauters: Investigator-initiated grants: Gilead, MSD, Pfizer; Consulting fees: Investigator-initiated grants from Gilead, Pfizer; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Speakers fees from Gilead, Pfizer; Support for attending meetings and/or travel: travel grants from Gilead and Pfizer. No potential financial or non-financial competing interest was reported by any of the other authors.
Figures


References
-
- Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., et al. WHO rapid evidence appraisal for COVID-19 therapies (REACT) working group. Association between Administration of Systemic Corticosteroids and Mortality among Critically ill Patients with COVID-19: a Meta-analysis. Jama. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

