Phase-change materials based on amorphous equichalcogenides
- PMID: 36801904
- PMCID: PMC9938898
- DOI: 10.1038/s41598-023-30160-7
Phase-change materials based on amorphous equichalcogenides
Abstract
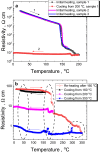
Phase-change materials, demonstrating a rapid switching between two distinct states with a sharp contrast in electrical, optical or magnetic properties, are vital for modern photonic and electronic devices. To date, this effect is observed in chalcogenide compounds based on Se, Te or both, and most recently in stoichiometric Sb2S3 composition. Yet, to achieve best integrability into modern photonics and electronics, the mixed S/Se/Te phase change medium is needed, which would allow a wide tuning range for such important physical properties as vitreous phase stability, radiation and photo-sensitivity, optical gap, electrical and thermal conductivity, non-linear optical effects, as well as the possibility of structural modification at nanoscale. In this work, a thermally-induced high-to-low resistivity switching below 200 °C is demonstrated in Sb-rich equichalcogenides (containing S, Se and Te in equal proportions). The nanoscale mechanism is associated with interchange between tetrahedral and octahedral coordination of Ge and Sb atoms, substitution of Te in the nearest Ge environment by S or Se, and Sb-Ge/Sb bonds formation upon further annealing. The material can be integrated into chalcogenide-based multifunctional platforms, neuromorphic computational systems, photonic devices and sensors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Kim T. Evolution of phase-change memory for the storage-class memory and beyond. IEEE Trans. Electron Devices. 2020;67:1394–1406. doi: 10.1109/TED.2020.2964640. - DOI
-
- Fantini P. Phase change memory applications: The history, the present and the future. J. Phys. D Appl. Phys. 2020;53:283002. doi: 10.1088/1361-6463/ab83ba. - DOI
-
- Zhang Y, Chou JB, Li J, Li H, Du Q, Yadav A, Zhou S, Shalaginov MY, Fang Zh, Zhong H, Roberts Ch, Robinson P, Bohlin B, Ríos C, Lin H, Kang M, Gu T, Warner J, Liberman V, Richardson K, Hu J. Broadband transparent optical phase change materials for high-performance nonvolatile photonics. Nat. Comm. 2019;10:4279. doi: 10.1038/s41467-019-12196-4. - DOI - PMC - PubMed
-
- Xu P, Zheng J, Doylend JK, Majumdar A. Low-loss and broadband nonvolatile phase-change directional coupler switches. ACS Photon. 2019;6:553–557. doi: 10.1021/acsphotonics.8b01628. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

