Corrosion damage and life prediction of concrete structure in the coking ammonium sulfate workshop of iron and steel industry
- PMID: 36801908
- PMCID: PMC9938225
- DOI: 10.1038/s41598-023-30015-1
Corrosion damage and life prediction of concrete structure in the coking ammonium sulfate workshop of iron and steel industry
Abstract
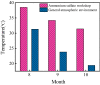
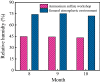
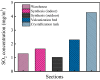
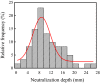
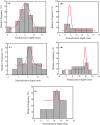
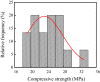
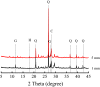
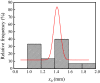
Iron and steel plants emit a large amount of CO2 and SO2 in the production process, and the high concentrations of acid gases lead to serious corrosion damage of concrete structures. In this paper, the environmental characteristics and corrosion damage degree of concrete in a 7-year-old coking ammonium sulfate workshop were investigated, and the neutralization life prediction of the concrete structure was carried out. Besides, the corrosion products were analyzed through concrete neutralization simulation test. The average temperature and relative humidity in the workshop were 34.7 °C and 43.4%, and they were 1.40 times higher and 1.70 times less than those of the general atmospheric environment, respectively. Both the concentrations of CO2 and SO2 were significantly different in various sections of the workshop, and they were much higher than those of the general atmospheric environment. The appearance corrosion and compressive strength loss of concrete were more serious in the sections with high SO2 concentration, such as vulcanization bed section and crystallization tank section. The neutralization depth of concrete in the crystallization tank section was the largest, with an average value of 19.86 mm. The corrosion products gypsum and CaCO3 were obviously visible in the surface layer of concrete, while only CaCO3 could be observed at 5 mm. The prediction model of concrete neutralization depth was established, and the remaining neutralization service life in the warehouse, synthesis section (indoor), synthesis section (outdoor), vulcanization bed section, and crystallization tank section were 69.21 a, 52.01 a, 88.56 a, 29.62 a, and 7.84 a, respectively.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Papadakis VG, Vayenas CG, Fardis MNA. Reaction problem of engineering approach to the concrete carbonation. ACIhE J. 1989;35(10):1639–1650.
-
- Papadakis VG, Vayenas CG, Fardis MN. Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem. Eng. Sci. 1991;46(5/6):1333–1338. doi: 10.1016/0009-2509(91)85060-B. - DOI
-
- Ishida T, Li CH. Modeling of carbonation based on thermo-hygro physics with strong coupling of mass transport and equilibrium in micro-pore structure of concrete. J. Adv. Concr. Technol. 2008;2(6):303–316. doi: 10.3151/jact.6.303. - DOI
-
- Gunasekara C, et al. Microstructure and strength development of quaternary blend high-volume fly ash concrete. J. Mater. Sci. 2020;55:6441–6456. doi: 10.1007/s10853-020-04473-1. - DOI
-
- Mainier FB, Almeida PCF, Nani B, Fernandes LH, Reis MF. Corrosion caused by sulfur dioxide in reinforced concrete. Open J. Civ. Eng. 2015;05(04):379–389. doi: 10.4236/ojce.2015.54038. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

