Vaccination Ameliorates Cellular Inflammatory Responses in SARS-CoV-2 Breakthrough Infections
- PMID: 36801946
- PMCID: PMC10304754
- DOI: 10.1093/infdis/jiad045
Vaccination Ameliorates Cellular Inflammatory Responses in SARS-CoV-2 Breakthrough Infections
Abstract
Background: Data on cellular immune responses in persons with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection following vaccination are limited. The evaluation of these patients with SARS-CoV-2 breakthrough infections may provide insight into how vaccinations limit the escalation of deleterious host inflammatory responses.
Methods: We conducted a prospective study of peripheral blood cellular immune responses to SARS-CoV-2 infection in 21 vaccinated patients, all with mild disease, and 97 unvaccinated patients stratified based on disease severity.
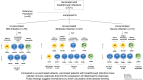
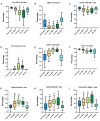
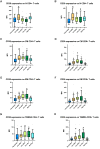
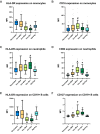
Results: We enrolled 118 persons (aged 50 years [SD 14.5 years], 52 women) with SARS-CoV-2 infection. Compared to unvaccinated patients, vaccinated patients with breakthrough infections had a higher percentage of antigen-presenting monocytes (HLA-DR+), mature monocytes (CD83+), functionally competent T cells (CD127+), and mature neutrophils (CD10+); and lower percentages of activated T cells (CD38+), activated neutrophils (CD64+), and immature B cells (CD127+CD19+). These differences widened with increased disease severity in unvaccinated patients. Longitudinal analysis showed that cellular activation decreased over time but persisted in unvaccinated patients with mild disease at 8-month follow-up.
Conclusions: Patients with SARS-CoV-2 breakthrough infections exhibit cellular immune responses that limit the progression of inflammatory responses and suggest mechanisms by which vaccination limits disease severity. These data may have implications for developing more effective vaccines and therapies. Clinical Trials Registration. NCT04401449.
Keywords: SARS-CoV-2; breakthrough infections; flow cytometry; immune response; vaccines.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2023.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

