Valine Treatment Enhances Antimicrobial Component Production in Mammary Epithelial Cells and the Milk of Lactating Goats Without Influencing the Tight Junction Barrier
- PMID: 36801983
- PMCID: PMC9938821
- DOI: 10.1007/s10911-023-09529-x
Valine Treatment Enhances Antimicrobial Component Production in Mammary Epithelial Cells and the Milk of Lactating Goats Without Influencing the Tight Junction Barrier
Erratum in
-
Correction to: Valine Treatment Enhances Antimicrobial Component Production in Mammary Epithelial Cells and the Milk of Lactating Goats Without Influencing the Tight Junction Barrier.J Mammary Gland Biol Neoplasia. 2023 Mar 14;28(1):5. doi: 10.1007/s10911-023-09533-1. J Mammary Gland Biol Neoplasia. 2023. PMID: 36929225 Free PMC article. No abstract available.
Abstract
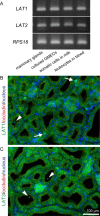
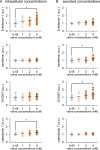
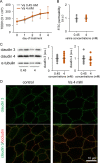
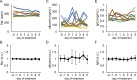
The production of antimicrobial components and the formation of less-permeable tight junctions (TJs) are important in the defense system of lactating mammary glands and for safe dairy production. Valine is a branched-chain amino acid that is actively consumed in the mammary glands and promotes the production of major milk components like β-casein; additionally, branched-chain amino acids stimulate antimicrobial component production in the intestines. Therefore, we hypothesized that valine strengthens the mammary gland defense system without influencing milk production. We investigated the effects of valine in vitro using cultured mammary epithelial cells (MECs) and in vivo using the mammary glands of lactating Tokara goats. Valine treatment at 4 mM increased the secretion of S100A7 and lactoferrin as well as the intracellular concentration of β-defensin 1 and cathelicidin 7 in cultured MECs. In addition, an intravenous injection of valine increased S100A7 levels in the milk of Tokara goats without influencing milk yield and milk components (i.e., fat, protein, lactose, and solids). In contrast, valine treatment did not affect TJ barrier function either in vitro or in vivo. These findings indicate that valine enhances antimicrobial component production without influencing milk production and TJ barrier function in lactating mammary glands; thus, valine contributes to safe dairy production.
Keywords: Antimicrobial component; Branched-chain amino acid; Mammary gland; Tight junction; Valine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Murphy JM. The genesis of bovine udder infection and mastitis; the occurrence of streptococcal infection in a cow population during a seven-year period and its relationship to age. Am J Vet Res. 1947;8:29–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

