Role of α6-Nicotinic Receptors in Alcohol-Induced GABAergic Synaptic Transmission and Plasticity to Cholinergic Interneurons in the Nucleus Accumbens
- PMID: 36802012
- PMCID: PMC10690621
- DOI: 10.1007/s12035-023-03263-5
Role of α6-Nicotinic Receptors in Alcohol-Induced GABAergic Synaptic Transmission and Plasticity to Cholinergic Interneurons in the Nucleus Accumbens
Abstract
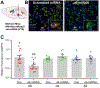
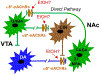
The prevailing view is that enhancement of dopamine (DA) transmission in the mesolimbic system, consisting of DA neurons in the ventral tegmental area (VTA) that project to the nucleus accumbens (NAc), underlies the reward properties of ethanol (EtOH) and nicotine (NIC). We have shown previously that EtOH and NIC modulation of DA release in the NAc is mediated by α6-containing nicotinic acetylcholine receptors (α6*-nAChRs), that α6*-nAChRs mediate low-dose EtOH effects on VTA GABA neurons and EtOH preference, and that α6*-nAChRs may be a molecular target for low-dose EtOH. However, the most sensitive target for reward-relevant EtOH modulation of mesolimbic DA transmission and the involvement of α6*-nAChRs in the mesolimbic DA reward system remains to be elucidated. The aim of this study was to evaluate EtOH effects on GABAergic modulation of VTA GABA neurons and VTA GABAergic input to cholinergic interneurons (CINs) in the NAc. Low-dose EtOH enhanced GABAergic input to VTA GABA neurons that was blocked by knockdown of α6*-nAChRs. Knockdown was achieved either by α6-miRNA injected into the VTA of VGAT-Cre/GAD67-GFP mice or by superfusion of the α-conotoxin MII[H9A;L15A] (MII). Superfusion of MII blocked EtOH inhibition of mIPSCs in NAc CINs. Concomitantly, EtOH enhanced CIN firing rate, which was blocked by knockdown of α6*-nAChRs with α6-miRNA injected into the VTA of VGAT-Cre/GAD67-GFP mice. The firing rate of CINs was not enhanced by EtOH in EtOH-dependent mice, and low-frequency stimulation (LFS; 1 Hz, 240 pulses) caused inhibitory long-term depression at this synapse (VTA-NAc CIN-iLTD) which was blocked by knockdown of α6*-nAChR and MII. Ethanol inhibition of CIN-mediated evoked DA release in the NAc was blocked by MII. Taken together, these findings suggest that α6*-nAChRs in the VTA-NAc pathway are sensitive to low-dose EtOH and play a role in plasticity associated with chronic EtOH.
Keywords: Alcohol; Alpha6 nAChRs (α6*-nAChRs); Cholinergic interneurons (CINs); Nicotine (NIC); Nicotinic acetylcholine receptors (nAChRs).
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures






Similar articles
-
α6 subunit-containing nicotinic receptors mediate low-dose ethanol effects on ventral tegmental area neurons and ethanol reward.Addict Biol. 2018 Sep;23(5):1079-1093. doi: 10.1111/adb.12559. Epub 2017 Sep 13. Addict Biol. 2018. PMID: 28901722 Free PMC article.
-
Functional nicotinic acetylcholine receptors containing α6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons.J Neurosci. 2011 Feb 16;31(7):2537-48. doi: 10.1523/JNEUROSCI.3003-10.2011. J Neurosci. 2011. PMID: 21325521 Free PMC article.
-
Modulation of dopamine release by ethanol is mediated by atypical GABAA receptors on cholinergic interneurons in the nucleus accumbens.Addict Biol. 2022 Jan;27(1):e13108. doi: 10.1111/adb.13108. Epub 2021 Oct 29. Addict Biol. 2022. PMID: 34713509 Free PMC article.
-
Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology.Acta Pharmacol Sin. 2009 Jun;30(6):740-51. doi: 10.1038/aps.2009.63. Acta Pharmacol Sin. 2009. PMID: 19498417 Free PMC article. Review.
-
VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems.Front Behav Neurosci. 2014 Jan 22;8:8. doi: 10.3389/fnbeh.2014.00008. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24478655 Free PMC article. Review.
Cited by
-
Alcohol-induced accumbal dopamine- and taurine release in female and male Wistar rats, an in vivo microdialysis study.J Neural Transm (Vienna). 2025 Jul;132(7):1051-1062. doi: 10.1007/s00702-025-02928-w. Epub 2025 Apr 18. J Neural Transm (Vienna). 2025. PMID: 40249403 Free PMC article.
-
Interleukin-10 enhances activity of ventral tegmental area dopamine neurons resulting in increased dopamine release.Brain Behav Immun. 2023 Oct;113:145-155. doi: 10.1016/j.bbi.2023.07.007. Epub 2023 Jul 13. Brain Behav Immun. 2023. PMID: 37453452 Free PMC article.
-
Catharanthine Modulates Mesolimbic Dopamine Transmission and Nicotine Psychomotor Effects via Inhibition of α6-Nicotinic Receptors and Dopamine Transporters.ACS Chem Neurosci. 2024 May 1;15(9):1738-1754. doi: 10.1021/acschemneuro.3c00478. Epub 2024 Apr 13. ACS Chem Neurosci. 2024. PMID: 38613458 Free PMC article.
-
Expression pattern of nicotinic acetylcholine receptor subunit transcripts in neurons and astrocytes in the ventral tegmental area and locus coeruleus.Eur J Neurosci. 2024 May;59(9):2225-2239. doi: 10.1111/ejn.16109. Epub 2023 Aug 4. Eur J Neurosci. 2024. PMID: 37539749 Free PMC article.
-
Ivermectin increases striatal cholinergic activity to facilitate dopamine terminal function.Cell Biosci. 2024 Apr 17;14(1):50. doi: 10.1186/s13578-024-01228-2. Cell Biosci. 2024. PMID: 38632622 Free PMC article.
References
-
- SAMHSA (2016) National Survey on Drug Use and Health (NSDUH)
-
- Sacks JJ et al. (2015) 2010 National and state costs of excessive alcohol consumption. Am J Prev Med 49(5):e73–e79 - PubMed
-
- Report SG, National center for chronic disease prevention and health promotion (US) Office on smoking and health (2014) The Health Consequences of Smoking—50 Years of Progress
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

