Baricitinib Safety for Events of Special Interest in Populations at Risk: Analysis from Randomised Trial Data Across Rheumatologic and Dermatologic Indications
- PMID: 36802049
- PMCID: PMC9939375
- DOI: 10.1007/s12325-023-02445-w
Baricitinib Safety for Events of Special Interest in Populations at Risk: Analysis from Randomised Trial Data Across Rheumatologic and Dermatologic Indications
Erratum in
-
Correction to: Baricitinib Safety for Events of Special Interest in Populations at Risk: Analysis from Randomised Trial Data Across Rheumatologic and Dermatologic Indications.Adv Ther. 2025 Sep;42(9):4717-4719. doi: 10.1007/s12325-025-03244-1. Adv Ther. 2025. PMID: 40643845 Free PMC article. No abstract available.
Abstract
Introduction: Baricitinib, a Janus kinase (JAK) 1/2 inhibitor, is an approved treatment for rheumatoid arthritis (RA), atopic dermatitis (AD), and alopecia areata (AA). Further characterisation of adverse events of special interest (AESI) for JAK inhibitors in at-risk populations will improve benefit-risk assessment for individual patients and diseases.
Methods: Data were pooled from clinical trials and long-term extensions in moderate-to-severe active RA, moderate-to-severe AD, and severe AA. Incidence rates (IR) per 100 patient-years of major adverse cardiovascular event (MACE), malignancy, venous thromboembolism (VTE), serious infection, and mortality were calculated for patients with low risk (younger than 65 years with no specified risk factors), and patients at risk (≥ 1 of: aged 65 years or older, atherosclerotic cardiovascular disease, diabetes mellitus, hypertension, current smoking, HDL cholesterol < 40 mg/dL, BMI ≥ 30 kg/m2, poor mobility on EQ-5D, or history of malignancy).
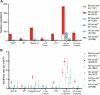
Results: Datasets included baricitinib exposure up to 9.3 years with 14,744 person-years of exposure (PYE) (RA), 3.9 years with 4628 PYE (AD), and 3.1 years with 1868 PYE (AA). In patients with low risk (RA: 31%, AD: 48%, AA: 49%), IRs for MACE (0.05, 0.04, 0), malignancies (0.20, 0.13, 0), VTE (0.09, 0.04, 0), serious infection (1.73, 1.18, 0.6), and mortality (0.04, 0, 0) in the RA, AD, and AA datasets, respectively, were low. In patients at risk (RA: 69%, AD: 52%, AA: 51%), IRs were for MACE (0.70, 0.25, 0.10), malignancies (1.23, 0.45, 0.31), VTE (0.66, 0.12, 0.10), serious infection (2.95, 2.30, 1.05), and mortality (0.78, 0.16, 0) for RA, AD, and AA datasets, respectively.
Conclusion: Populations with low risk have low incidence of the examined JAK inhibitor-related AESI. In the dermatologic indications, incidence is also low for patients at risk. Considering individual disease burden, risk factors, and response to treatment is relevant to make informed decisions for individual patients treated with baricitinib.
Keywords: Adverse events; Baricitinib; JAK inhibitors; MACE; Malignancy; Mortality; Safety; Serious infection; VTE.
© 2023. The Author(s).
Conflict of interest statement
Peter C. Taylor has been a consultant for or has received grant/research support from AbbVie, Biogen, Celgene, Eli Lilly and Company, Galapagos NV, Gilead Sciences, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sandoz, and UCB Pharma.
Thomas Bieber was speaker or consultant or Investigator for AbbVie, Affibody, Allmiral, AnaptysBio, Arena, Asana Biosciences, ASLAN pharma, Bayer Health, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Connect Pharma, Dermavant, Domain Therapeutics, EQRx, Eli Lilly and Company, Galderma, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, L´Oréal, MSD, Novartis, Numab, OM-Pharma, Pfizer, Pierre Fabre, Q32bio, RAPT, Sanofi/Regeneron, UCB. He is founder and chairman of the board of the non-profit biotech “Davos Biosciences”.
Rieke Alten has been a consultant for or received grant or research support from AbbVie, Bristol Myers Squibb, Celltrion, Chugai, Eli Lilly and Company, Galapagos NV, Gilead Sciences, Janssen, Novartis, Pfizer, Roche, and UCB Pharma.
Torsten Witte has received grant/research support or received honoraria or support for attending meetings or participated on a data safety monitoring board or advisory board for AbbVie, Chugai, Eli Lilly and Company, Galapagos, Medac, Janssen, Novartis, Pfizer, Roche, and UCB Pharma.
James Galloway has been a consultant for or has received grant/research support or received honoraria from AbbVie, Eli Lilly and Company, Galapagos, and Pfizer.
Walter Deberdt, Maher Issa, Ewa Haladyj, Inmaculada De La Torre, and Susanne Grond are employees and shareholders of Eli Lilly and Company.
Andreas Wollenberg has served as an advisor or paid speaker for or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Beiersdorf, Bioderma, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Galapagos, Galderma, Glenmark, Janssen-Cilag, L’Oreal, LEO Pharma, Maruho, MedImmune/AstraZeneca, Merck, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi-Aventis/Genzyme, and UCB.
Figures
References
-
- Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–9. - PubMed
-
- Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. - PubMed
-
- Bruin-Weller MD, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

