Plasma Gelsolin Enhances Phagocytosis of Candida auris by Human Neutrophils through Scavenger Receptor Class B
- PMID: 36802172
- PMCID: PMC10101141
- DOI: 10.1128/spectrum.04082-22
Plasma Gelsolin Enhances Phagocytosis of Candida auris by Human Neutrophils through Scavenger Receptor Class B
Abstract
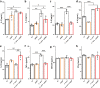
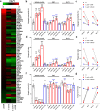
In addition to its role as an actin-depolymerizing factor in the blood, plasma gelsolin (pGSN) binds bacterial molecules and stimulates the phagocytosis of bacteria by macrophages. Here, using an in vitro system, we assessed whether pGSN could also stimulate phagocytosis of the fungal pathogen Candida auris by human neutrophils. The extraordinary ability of C. auris to evade immune responses makes it particularly challenging to eradicate in immunocompromised patients. We demonstrate that pGSN significantly enhances C. auris uptake and intracellular killing. Stimulation of phagocytosis was accompanied by decreased neutrophil extracellular trap (NET) formation and reduced secretion of proinflammatory cytokines. Gene expression studies revealed pGSN-dependent upregulation of scavenger receptor class B (SR-B). Inhibition of SR-B using sulfosuccinimidyl oleate (SSO) and block lipid transport-1 (BLT-1) decreased the ability of pGSN to enhance phagocytosis, indicating that pGSN potentiates the immune response through an SR-B-dependent pathway. These results suggest that the response of the host's immune system during C. auris infection may be enhanced by the administration of recombinant pGSN. IMPORTANCE The incidence of life-threatening multidrug-resistant Candida auris infections is rapidly growing, causing substantial economic costs due to outbreaks in hospital wards. Primary and secondary immunodeficiencies in susceptible individuals, such as those with leukemia, solid organ transplants, diabetes, and ongoing chemotherapy, often correlate with decreased plasma gelsolin concentration (hypogelsolinemia) and impairment of innate immune responses due to severe leukopenia. Immunocompromised patients are predisposed to superficial and invasive fungal infections. Morbidity caused by C. auris among immunocompromised patients can be as great as 60%. In the era of ever-growing fungal resistance in an aging society, it is critical to seek novel immunotherapies that may help combat these infections. The results reported here suggest the possibility of using pGSN as an immunomodulator of the immune response by neutrophils during C. auris infection.
Keywords: Candida auris; inflammation; neutrophils; phagocytosis; plasma gelsolin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bucki R, Kulakowska A, Byfield FJ, Zendzian-Piotrowska M, Baranowski M, Marzec M, Winer JP, Ciccarelli NJ, Górski J, Drozdowski W, Bittman R, Janmey PA. 2010. Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am J Physiol Cell Physiol 299:C1516–C1523. doi: 10.1152/ajpcell.00051.2010. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

