Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions
- PMID: 36802199
- PMCID: PMC10330013
- DOI: 10.1002/adhm.202203148
Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions
Abstract
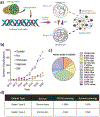
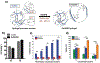
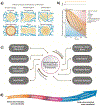
Gelatin is a widely utilized bioprinting biomaterial due to its cell-adhesive and enzymatically cleavable properties, which improve cell adhesion and growth. Gelatin is often covalently cross-linked to stabilize bioprinted structures, yet the covalently cross-linked matrix is unable to recapitulate the dynamic microenvironment of the natural extracellular matrix (ECM), thereby limiting the functions of bioprinted cells. To some extent, a double network bioink can provide a more ECM-mimetic, bioprinted niche for cell growth. More recently, gelatin matrices are being designed using reversible cross-linking methods that can emulate the dynamic mechanical properties of the ECM. This review analyzes the progress in developing gelatin bioink formulations for 3D cell culture, and critically analyzes the bioprinting and cross-linking techniques, with a focus on strategies to optimize the functions of bioprinted cells. This review discusses new cross-linking chemistries that recapitulate the viscoelastic, stress-relaxing microenvironment of the ECM, and enable advanced cell functions, yet are less explored in engineering the gelatin bioink. Finally, this work presents the perspective on the areas of future research and argues that the next generation of gelatin bioinks should be designed by considering cell-matrix interactions, and bioprinted constructs should be validated against currently established 3D cell culture standards to achieve improved therapeutic outcomes.
Keywords: 3D bioprinting; covalent cross-linking; extracellular matrix; gelatin bioinks; stress relaxation; viscoelasticity.
© 2023 Wiley-VCH GmbH.
Figures



References
-
- Lewis A, Koukoura A, Tsianos G-I, Gargavanis AA, Nielsen AA, Vassiliadis E, Organ donation in the US and Europe: The supply vs demand imbalance, Transplantation Reviews 35(2) (2021) 100585. - PubMed
-
- Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, Wertheim JA, Rothblatt M, Boyden ES, Eidbo E, Lee WPA, Pomahac B, Brandacher G, Weinstock DM, Elliott G, Nelson D, Acker JP, Uygun K, Schmalz B, Weegman BP, Tocchio A, Fahy GM, Storey KB, Rubinsky B, Bischof J, Elliott JAW, Woodruff TK, Morris GJ, Demirci U, Brockbank KGM, Woods EJ, Ben RN, Baust JG, Gao D, Fuller B, Rabin Y, Kravitz DC, Taylor MJ, Toner M, The promise of organ and tissue preservation to transform medicine, Nat Biotechnol 35(6) (2017) 530–542. - PMC - PubMed
-
- Hunsberger J, Neubert J, Wertheim JA, Allickson J, Atala A, Bioengineering Priorities on a Path to Ending Organ Shortage, Current Stem Cell Reports 2(2) (2016) 118–127.
-
- Langer R, Vacanti JP, Tissue engineering, Science 260(5110) (1993) 920–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

