Cost-Analysis of Subcutaneous vs Intravenous Administration of Natalizumab Based on Patient Care Pathway in Multiple Sclerosis in Spain
- PMID: 36802327
- PMCID: PMC10169937
- DOI: 10.1007/s41669-023-00394-2
Cost-Analysis of Subcutaneous vs Intravenous Administration of Natalizumab Based on Patient Care Pathway in Multiple Sclerosis in Spain
Abstract
Introduction: A subcutaneous (SC) formulation of natalizumab has been recently authorised for multiple sclerosis patients. This study aimed to assess the implications of the new SC formulation, and to compare the annual treatment costs of SC versus intravenous (IV) natalizumab therapy from both the Spanish healthcare system (direct health cost) and the patient (indirect cost) perspectives.
Methods: A patient care pathway map and a cost-minimisation analysis were developed to estimate SC and IV natalizumab annual costs over a 2-year time horizon. Considering the patient care pathway and according to natalizumab experience (IV) or estimation (SC), a national expert panel involving neurologists, pharmacists, and nurses provided information/data regarding resource consumption for drug and patient preparation, administration, and documentation. One hour of observation was applied to the first six (SC) or 12 (IV) doses, and 5 min for successive doses. The Day hospital (infusion suite) facilities at a reference hospital were considered for IV administrations and the first six SC injections. For successive SC injections, either a reference hospital or regional hospital in a consulting room was considered. Productivity time associated with travel (56 min to reference hospital, 24 min to regional hospital) and waiting time pre- and post-treatment (SC 15 min, IV 25 min) were assessed for patients and caregivers (accompanying 20% of SC and 35% of IV administrations). National salaries for healthcare professionals were used for cost estimation (€, year 2021).
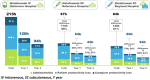
Results: At years 1 and 2, total time and cost savings (excluding drug acquisition cost) per patient, driven by saving on administration and patient and caregiver productivity for SC at a reference hospital versus IV at a reference hospital, were 116 h (a reduction of 54.6%) and €3682.82 (a reduction of 66.2%). In the case of natalizumab SC at a regional hospital, the total time and cost saving were 129 h (a reduction of 60.6%) and €3883.47 (a reduction of 69.8%).
Conclusions: Besides the potential benefits of convenient administration and improving work-life balance, as suggested by the expert panel, natalizumab SC was associated with cost savings for the healthcare system by avoiding drug preparation, reducing administration time, and freeing up infusion suite capacity. Additional cost savings could be derived with regional hospital administration of natalizumab SC by reducing productivity loss.
© 2023. The Author(s).
Conflict of interest statement
Ana María Alonso Torres has received compensation for serving on advisory boards for Biogen Spain S.L.U., Bristol Myers Squibb (BMS), Janssen, Novartis, Roche, and Sanofi; speaker honoraria from Almirall, Biogen Spain S.L.U., BMS, Janssen, Merck, Novartis, Roche, and Sanofi. Ángel Guillermo Arévalo Bernabé has received compensation for serving on advisory boards for Biogen Spain S.L.U. and Merck. Noelia Becerril Ríos has received compensation for serving on advisory boards and speaker fees from Almirall, Bayer, Biogen Spain S.L.U., BMS, Janssen, Merck, Novartis, and Sanofi. María Fuensanta Hellín Gil has received compensation for serving on advisory boards and a speaker event for Biogen Spain S.L.U. José Manuel Martínez Sesmero has received compensation for serving on advisory boards for Biogen Spain S.L.U., Merck, Roche, and Teva Pharmaceutical. Virginia Meca Lallana has received compensation for serving on scientific advisory boards and has received speaker honoraria from Almirall, Biogen Spain S.L.U., BMS, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Terumo, and Teva Pharmaceutical. Lluís Ramió-Torrentá has received compensation for serving on advisory boards and speaker honoraria from Almirall, Biogen Spain S.L.U., BMS, Merck, Novartis, Roche, Sanofi, and Teva Pharmaceutical. Alfredo Rodriguez-Antigüedad Zarranz has received compensation for serving on advisory boards or speaker honoraria from Biogen, BMS, Janssen, Merck‐Serono, Novartis, Roche, and Sanofi. Laura Gómez Maldonado and Inés Triana Junco are employees of Biogen Spain S.L.U. and hold shares or stocks as part of their remuneration. Manuel Gómez-Barrera, Nataly Espinoza Cámac, and Itziar Oyagüez work for Pharmacoeconomics & Outcomes Research Iberia (PORIB), an independent research organisation, which received funding pursuant to a contract with Biogen Spain S.L.U.
Figures
References
-
- García Merino A, Ramón Ara Callizo J, Fernández Fernández O, Landete Pascual L, Moral Torres E, Rodríguez-Antigüedad Zarrantz A. Consensus statement on the treatment of multiple sclerosis by the Spanish Society of Neurology in 2016. Neurol Barc Spain. 2017;32:113–119. doi: 10.1016/j.nrl.2016.02.026. - DOI - PubMed
LinkOut - more resources
Full Text Sources