Consonance Perception in Congenital Amusia: Behavioral and Brain Responses to Harmonicity and Beating Cues
- PMID: 36802367
- PMCID: PMC10117172
- DOI: 10.1162/jocn_a_01973
Consonance Perception in Congenital Amusia: Behavioral and Brain Responses to Harmonicity and Beating Cues
Abstract
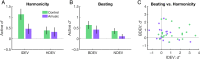
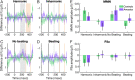
Congenital amusia is a neurodevelopmental disorder characterized by difficulties in the perception and production of music, including the perception of consonance and dissonance, or the judgment of certain combinations of pitches as more pleasant than others. Two perceptual cues for dissonance are inharmonicity (the lack of a common fundamental frequency between components) and beating (amplitude fluctuations produced by close, interacting frequency components). Amusic individuals have previously been reported to be insensitive to inharmonicity, but to exhibit normal sensitivity to beats. In the present study, we measured adaptive discrimination thresholds in amusic participants and found elevated thresholds for both cues. We recorded EEG and measured the MMN in evoked potentials to consonance and dissonance deviants in an oddball paradigm. The amplitude of the MMN response was similar overall for amusic and control participants; however, in controls, there was a tendency toward larger MMNs for inharmonicity than for beating cues, whereas the opposite tendency was observed for the amusic participants. These findings suggest that initial encoding of consonance cues may be intact in amusia despite impaired behavioral performance, but that the relative weight of nonspectral (beating) cues may be increased for amusic individuals.
© 2023 Massachusetts Institute of Technology.
Figures





References
-
- Albouy, P., Caclin, A., Norman-Haignere, S. V., Lévêque, Y., Peretz, I., Tillmann, B., et al. (2019). Decoding task-related functional brain imaging data to identify developmental disorders: The case of congenital amusia. Frontiers in Neuroscience, 13, 1165. 10.3389/fnins.2019.01165, - DOI - PMC - PubMed

