Spontaneous nucleation and fast aggregate-dependent proliferation of α-synuclein aggregates within liquid condensates at neutral pH
- PMID: 36802433
- PMCID: PMC9992821
- DOI: 10.1073/pnas.2208792120
Spontaneous nucleation and fast aggregate-dependent proliferation of α-synuclein aggregates within liquid condensates at neutral pH
Abstract
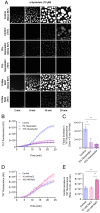
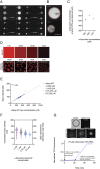
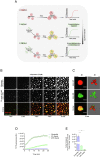
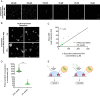
The aggregation of α-synuclein into amyloid fibrils has been under scrutiny in recent years because of its association with Parkinson's disease. This process can be triggered by a lipid-dependent nucleation process, and the resulting aggregates can proliferate through secondary nucleation under acidic pH conditions. It has also been recently reported that the aggregation of α-synuclein may follow an alternative pathway, which takes place within dense liquid condensates formed through phase separation. The microscopic mechanism of this process, however, remains to be clarified. Here, we used fluorescence-based assays to enable a kinetic analysis of the microscopic steps underlying the aggregation process of α-synuclein within liquid condensates. Our analysis shows that at pH 7.4, this process starts with spontaneous primary nucleation followed by rapid aggregate-dependent proliferation. Our results thus reveal the microscopic mechanism of α-synuclein aggregation within condensates through the accurate quantification of the kinetic rate constants for the appearance and proliferation of α-synuclein aggregates at physiological pH.
Keywords: Parkinson’s disease; phase separation; protein condensates.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Balestrino R., Schapira A., Parkinson disease. Eur. J. Neurol. 27, 27–42 (2020). - PubMed
-
- Goedert M., Spillantini M. G., Del Tredici K., Braak H., 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13 (2013). - PubMed
-
- Shahmoradian S. H., et al. , Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 22, 1099–1109 (2019). - PubMed
-
- Spillantini M. G., et al. , α-synuclein in Lewy bodies. Nature 388, 839–840 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

