Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial
- PMID: 36803273
- PMCID: PMC9940050
- DOI: 10.1186/s13063-023-07114-5
Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial
Abstract
Introduction: The present study aimed to determine the impact of vitamin D supplementation (VDs) on recovery delay among COVID-19 patients.
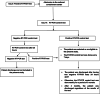
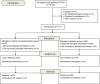
Methods: We performed a randomized controlled clinical trial at the national COVID-19 containment center in Monastir (Tunisia), from May to August 2020. Simple randomization was done in a 1:1 allocation ratio. We included patients aged more than 18 years who had confirmed reverse transcription-polymerase chain reaction (RT-PCR) and who remained positive on the 14th day. The intervention group received VDs (200,000 IU/1 ml of cholecalciferol); the control group received a placebo treatment (physiological saline (1 ml)). We measured the recovery delay and the cycle threshold (Ct) values in RT-PCR for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The log-rank test and hazard ratios (HR) were calculated.
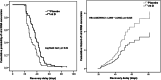
Results: A total of 117 patients were enrolled. The mean age was 42.7 years (SD 14). Males represented 55.6%. The median duration of viral RNA conversion was 37 days (95% confidence interval (CI): 29-45.50) in the intervention group and 28 days (95% CI: 23-39) in the placebo group (p=0.010). HR was 1.58 (95% CI: 1.09-2.29, p=0.015). Ct values revealed a stable trend over time in both groups.
Conclusion: VDs was not associated with a shortened recovery delay when given to patients for whom the RT-PCR remained positive on the 14th day.
Trial registration: This study was approved by the Human Subjects Protection Tunisia center (TN2020-NAT-INS-40) on April 28, 2020, and by ClinicalTrial.gov on May 12, 2021 with approval number ClinicalTrials.gov ID: NCT04883203 .
Keywords: Adult; Convalescence; Infection; SARS-CoV-2; Tunisia; Vitamins.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Grove A, Osokogu O, Al-Khudairy L, Mehrabian A, Zanganeh M, Brown A, et al. Association between vitamin D supplementation or serum vitamin D level and susceptibility to SARS-CoV-2 infection or COVID-19 including clinical course, morbidity and mortality outcomes? A systematic review. BMJ Open. 2021;11(5):e043737. doi: 10.1136/bmjopen-2020-043737. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

