Redox regulation of KV7 channels through EF3 hand of calmodulin
- PMID: 36803414
- PMCID: PMC9988260
- DOI: 10.7554/eLife.81961
Redox regulation of KV7 channels through EF3 hand of calmodulin
Abstract
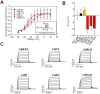
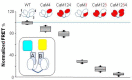
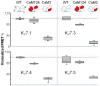
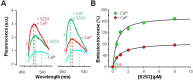
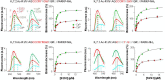
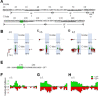
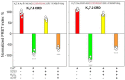
Neuronal KV7 channels, important regulators of cell excitability, are among the most sensitive proteins to reactive oxygen species. The S2S3 linker of the voltage sensor was reported as a site-mediating redox modulation of the channels. Recent structural insights reveal potential interactions between this linker and the Ca2+-binding loop of the third EF-hand of calmodulin (CaM), which embraces an antiparallel fork formed by the C-terminal helices A and B, constituting the calcium responsive domain (CRD). We found that precluding Ca2+ binding to the EF3 hand, but not to EF1, EF2, or EF4 hands, abolishes oxidation-induced enhancement of KV7.4 currents. Monitoring FRET (Fluorescence Resonance Energy Transfer) between helices A and B using purified CRDs tagged with fluorescent proteins, we observed that S2S3 peptides cause a reversal of the signal in the presence of Ca2+ but have no effect in the absence of this cation or if the peptide is oxidized. The capacity of loading EF3 with Ca2+ is essential for this reversal of the FRET signal, whereas the consequences of obliterating Ca2+ binding to EF1, EF2, or EF4 are negligible. Furthermore, we show that EF3 is critical for translating Ca2+ signals to reorient the AB fork. Our data are consistent with the proposal that oxidation of cysteine residues in the S2S3 loop relieves KV7 channels from a constitutive inhibition imposed by interactions between the EF3 hand of CaM which is crucial for this signaling.
Keywords: E. coli; EF-hand; KCNQ; calcium; calmodulin; cell biology; neuroscience; redox; signal transduction.
© 2023, Nuñez et al.
Conflict of interest statement
EN, FJ, AM, JU, AA, CM, GB, CD, OM, NG, AV No competing interests declared
Figures












Update of
References
-
- Archer CR, Enslow BT, Taylor AB, De la Rosa V, Bhattacharya A, Shapiro MS. A mutually induced conformational fit underlies ca2+-directed interactions between calmodulin and the proximal C terminus of KCNQ4 K+ channels. The Journal of Biological Chemistry. 2019;294:6094–6112. doi: 10.1074/jbc.RA118.006857. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

