A bat MERS-like coronavirus circulates in pangolins and utilizes human DPP4 and host proteases for cell entry
- PMID: 36803605
- PMCID: PMC9933427
- DOI: 10.1016/j.cell.2023.01.019
A bat MERS-like coronavirus circulates in pangolins and utilizes human DPP4 and host proteases for cell entry
Abstract
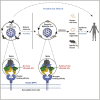
It is unknown whether pangolins, the most trafficked mammals, play a role in the zoonotic transmission of bat coronaviruses. We report the circulation of a novel MERS-like coronavirus in Malayan pangolins, named Manis javanica HKU4-related coronavirus (MjHKU4r-CoV). Among 86 animals, four tested positive by pan-CoV PCR, and seven tested seropositive (11 and 12.8%). Four nearly identical (99.9%) genome sequences were obtained, and one virus was isolated (MjHKU4r-CoV-1). This virus utilizes human dipeptidyl peptidase-4 (hDPP4) as a receptor and host proteases for cell infection, which is enhanced by a furin cleavage site that is absent in all known bat HKU4r-CoVs. The MjHKU4r-CoV-1 spike shows higher binding affinity for hDPP4, and MjHKU4r-CoV-1 has a wider host range than bat HKU4-CoV. MjHKU4r-CoV-1 is infectious and pathogenic in human airways and intestinal organs and in hDPP4-transgenic mice. Our study highlights the importance of pangolins as reservoir hosts of coronaviruses poised for human disease emergence.
Keywords: bat MERS-like coronavirus; dipeptidyl peptidase-4; furin cleavage site; pangolin.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






Comment in
-
Pangolin merbecovirus gets down to (poly)basics.Cell. 2023 Feb 16;186(4):688-690. doi: 10.1016/j.cell.2023.01.009. Cell. 2023. PMID: 36803601 Free PMC article.
References
-
- Hu B., Zeng L.-P., Yang X.-L., Ge X.-Y., Zhang W., Li B., Xie J.-Z., Shen X.-R., Zhang Y.-Z., Wang N., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLOS Pathog. 2017;13:e1006698. doi: 10.1371/journal.ppat.1006698. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

