Patient-Reported Symptom and Health-Related Quality-of-Life Validation and Responsiveness During the First 6 Months of Treatment for Mycobacterium avium Complex Pulmonary Disease
- PMID: 36803647
- PMCID: PMC10329268
- DOI: 10.1016/j.chest.2023.02.015
Patient-Reported Symptom and Health-Related Quality-of-Life Validation and Responsiveness During the First 6 Months of Treatment for Mycobacterium avium Complex Pulmonary Disease
Abstract
Background: Nontuberculous mycobacteria (NTM), predominately Mycobacterium avium complex (MAC), cause chronic pulmonary disease. Improvements in symptoms and health-related quality of life (HRQoL) are important treatment outcomes, but no validated patient-reported outcome (PRO) measure exists.
Research question: What are the validity and responsiveness of the Quality of Life-Bronchiectasis (QOL-B) questionnaire respiratory symptoms scale and key HRQoL measures during the first 6 months of MAC pulmonary disease (MAC-PD) treatment?
Study design and methods: Comparison of Two- vs Three-antibiotic Therapy for Pulmonary Mycobacterium Avium Complex Disease (MAC2v3) is an ongoing randomized, multisite pragmatic clinical trial. Patients with MAC-PD were randomized to azithromycin-based two-drug or three-drug therapy; treatment groups were combined for this analysis. PROs were measured at baseline, 3 months, and 6 months. The QOL-B respiratory symptoms, vitality, physical functioning, health perceptions, and NTM symptom domain scores (on a scale of 0-100, with 100 being best) were analyzed separately. We performed psychometric and descriptive analyses in the population enrolled as of the time of analysis and calculated the minimal important difference (MID) using distribution-based methods. Finally, we evaluated responsiveness using paired t tests and latent growth curve analysis in the subset with longitudinal surveys completed by the time of analysis.
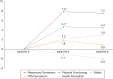
Results: The baseline population included 228 patients, of whom 144 had completed longitudinal surveys. Patients predominately were female (82%) and had bronchiectasis (88%); 50% were 70 years of age or older. The respiratory symptoms domain showed good psychometric properties (no floor or ceiling effects; Cronbach's α, 0.85) and an MID of 6.4 to 6.9. Vitality and health perceptions domain scores performed similarly. Respiratory symptoms domain scores improved by 7.8 points (P < .0001) and 7.5 points (P < .0001), and the physical functioning domain score improved by 4.6 points (P < .003) and 4.2 points (P = .01) at 3 and 6 months, respectively. Latent growth curve analysis confirmed a nonlinear, statistically significant improvement in respiratory symptoms and physical functioning domain scores by 3 months.
Interpretation: The QOL-B respiratory symptoms and physical functioning scales exhibited good psychometric properties in patients with MAC-PD. Respiratory symptoms scores improved beyond the MID by 3 months after treatment initiation.
Trial registry: ClinicalTrials.gov; No.: NCT03672630; URL: www.
Clinicaltrials: gov.
Keywords: Mycobacterium avium complex; patient-reported outcome measures.
Copyright © 2023 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- US Food and Drug Administration, Center for Drug Evaluation and Research (CDER) The voice of the patient. Non-tuberculous mycobacterial (NTM) lung infection. http://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFe... Public Meeting: October 15, 2015. Report Date: April, 2016. US Food and Drug Administration website.
-
- US Food and Drug Administration, Center for Drug Evaluation and Research. Draft guidance for industry: nontuberculous mycobacterial pulmonary disease caused by Mycobacterium avium complex: developing drugs for treatment. 2021. Accessed March 3, 2023. https://www.fda.gov/media/152501/download
-
- Griffith D.E., Aksamit T., Brown-Elliott B.A., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. - PubMed
-
- Koh W.J., Moon S.M., Kim S.Y., et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J. 2017;50(3):1602503. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

