Rediscovery of poly(ethylene glycol)s as a cryoprotectant for mesenchymal stem cells
- PMID: 36803669
- PMCID: PMC9942331
- DOI: 10.1186/s40824-023-00356-z
Rediscovery of poly(ethylene glycol)s as a cryoprotectant for mesenchymal stem cells
Abstract
Background: A medium containing dimethyl sulfoxide (DMSO) (10% v/v) is most widely used for cell cryopreservation at -196 °C. However, residual DMSO consistently raises concerns because of its toxicity; thus, its complete removal process is required.
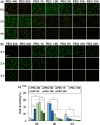
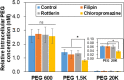
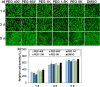
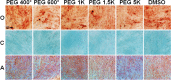
Method: As biocompatible polymers approved by the Food and Drug Administration for various biomedical applications for humans, poly(ethylene glycol)s (PEGs) with various molecular weights (400, 600, 1 K, 1.5 K, 5 K, 10 K, and 20 K Da) were studied as a cryoprotectant of mesenchymal stem cells (MSCs). Considering the cell permeability difference of PEGs depending on their molecular weight, the cells were preincubated for 0 h (no incubation), 2 h, and 4 h at 37 °C in the presence of PEGs at 10 wt.% before cryopreservation at -196 °C for 7 days. Then, cell recovery was assayed.
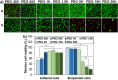
Results: We found that low molecular weight PEGs (400 and 600 Da) exhibit excellent cryoprotecting properties by 2 h preincubation, whereas intermediate molecular weight PEGs (1 K, 1.5 K, and 5 K Da) exhibit their cryoprotecting properties without preincubation. High molecular weight PEGs (10 K and 20 K Da) were ineffective as cryoprotectants for MSCs. Studies on ice recrystallization inhibition (IRI), ice nucleation inhibition (INI), membrane stabilization, and intracellular transport of PEGs suggest that low molecular weight PEGs (400 and 600 Da) exhibit excellent intracellular transport properties, and thus the internalized PEGs during preincubation contribute to the cryoprotection. Intermediate molecular weight PEGs (1 K, 1.5 K, and 5 K Da) worked by extracellular PEGs through IRI, INI, as well as partly internalized PEGs. High molecular weight PEGs (10 K and 20 K Da) killed the cells during preincubation and were ineffective as cryoprotectants.
Conclusions: PEGs can be used as cryoprotectants. However, the detailed procedures, including preincubation, should consider the effect of the molecular weight of PEGs. The recovered cells well proliferated and underwent osteo/chondro/adipogenic differentiation similar to the MSCs recovered from the traditional DMSO 10% system.
Keywords: Cryoprotection; Ice recrystallization; Molecular weight; Permeability; Poly(ethylene glycol).
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Low-Molecular-Weight PEGs for Cryopreservation of Stem Cell Spheroids.Biomater Res. 2024 Jun 6;28:0037. doi: 10.34133/bmr.0037. eCollection 2024. Biomater Res. 2024. PMID: 38845843 Free PMC article.
-
Size and Shape Control of Ice Crystals by Amphiphilic Block Copolymers and Their Implication in the Cryoprotection of Mesenchymal Stem Cells.ACS Appl Mater Interfaces. 2021 Jul 28;13(29):33969-33980. doi: 10.1021/acsami.1c09933. Epub 2021 Jul 18. ACS Appl Mater Interfaces. 2021. PMID: 34275265
-
Poly(l-alanine-co-l-lysine)-g-Trehalose as a Biomimetic Cryoprotectant for Stem Cells.Biomacromolecules. 2022 May 9;23(5):1995-2006. doi: 10.1021/acs.biomac.1c01701. Epub 2022 Apr 12. Biomacromolecules. 2022. PMID: 35412815
-
Final report of the amended safety assessment of PEG-5, -10, -16, -25, -30, and -40 soy sterol.Int J Toxicol. 2004;23 Suppl 2:23-47. doi: 10.1080/10915810490499046. Int J Toxicol. 2004. PMID: 15513823 Review.
-
Effect of developmental stage on bovine oocyte plasma membrane water and cryoprotectant permeability characteristics.Mol Reprod Dev. 1998 Apr;49(4):408-15. doi: 10.1002/(SICI)1098-2795(199804)49:4<408::AID-MRD8>3.0.CO;2-R. Mol Reprod Dev. 1998. PMID: 9508092 Review.
Cited by
-
Low-Molecular-Weight PEGs for Cryopreservation of Stem Cell Spheroids.Biomater Res. 2024 Jun 6;28:0037. doi: 10.34133/bmr.0037. eCollection 2024. Biomater Res. 2024. PMID: 38845843 Free PMC article.
-
Sphingosine-1-phosphate Treatment Improves Cryopreservation Efficiency in Human Mesenchymal Stem Cells.Life (Basel). 2023 May 30;13(6):1286. doi: 10.3390/life13061286. Life (Basel). 2023. PMID: 37374070 Free PMC article.
-
Polyelectrolytes Are Effective Cryoprotectants for Extracellular Vesicles.ACS Appl Mater Interfaces. 2024 Dec 25;16(51):70174-70186. doi: 10.1021/acsami.4c11852. Epub 2024 Dec 12. ACS Appl Mater Interfaces. 2024. PMID: 39667739 Free PMC article.
-
The Art of PEGylation: From Simple Polymer to Sophisticated Drug Delivery System.Int J Mol Sci. 2025 Mar 27;26(7):3102. doi: 10.3390/ijms26073102. Int J Mol Sci. 2025. PMID: 40243857 Free PMC article. Review.
-
Overcoming ice: cutting-edge materials and advanced strategies for effective cryopreservation of biosample.J Nanobiotechnology. 2025 Mar 7;23(1):187. doi: 10.1186/s12951-025-03265-6. J Nanobiotechnology. 2025. PMID: 40050919 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

