Health technology assessment of diagnostic tests: a state of the art review of methods guidance from international organizations
- PMID: 36803886
- PMCID: PMC7614237
- DOI: 10.1017/S0266462323000065
Health technology assessment of diagnostic tests: a state of the art review of methods guidance from international organizations
Abstract
Objectives: To identify which international health technology assessment (HTA) agencies are undertaking evaluations of medical tests, summarize commonalities and differences in methodological approach, and highlight examples of good practice.
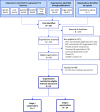
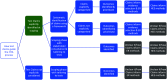
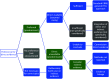
Methods: A methodological review incorporating: systematic identification of HTA guidance documents mentioning evaluation of tests; identification of key contributing organizations and abstraction of approaches to all essential HTA steps; summary of similarities and differences between organizations; and identification of important emergent themes which define the current state of the art and frontiers where further development is needed.
Results: Seven key organizations were identified from 216 screened. The main themes were: elucidation of claims of test benefits; attitude to direct and indirect evidence of clinical effectiveness (including evidence linkage); searching; quality assessment; and health economic evaluation. With the exception of dealing with test accuracy data, approaches were largely based on general approaches to HTA with few test-specific modifications. Elucidation of test claims and attitude to direct and indirect evidence are where we identified the biggest dissimilarities in approach.
Conclusions: There is consensus on some aspects of HTA of tests, such as dealing with test accuracy, and examples of good practice which HTA organizations new to test evaluation can emulate. The focus on test accuracy contrasts with universal acknowledgment that it is not a sufficient evidence base for test evaluation. There are frontiers where methodological development is urgently required, notably integrating direct and indirect evidence and standardizing approaches to evidence linkage.
Keywords: diagnostic tests; health technology assessment; methods.
Conflict of interest statement
The authors declare none.
Figures
References
-
- Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making. 1991;11:88–94. - PubMed
-
- Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, Bossuyt PMM, Deeks JJ. Assessing the value of diagnostic tests: A framework for designing and evaluating trials. BMJ. 2012;344:e686. - PubMed
-
- Bossuyt PM, Lijmer JG. Traditional health outcomes in the evaluation of diagnostic tests. Acad Radiol. 1999;6:S77–S80; discussion S3-4. - PubMed
-
- Fineberg HV. Evaluation of computed tomography: achievement and challenge. AJR Am J Roentgenol. 1978;131:1–4. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

