Efficacy and safety of Obex® in overweight and obese subjects: a randomised, double-blind, placebo-controlled clinical trial
- PMID: 36804035
- PMCID: PMC9940432
- DOI: 10.1186/s12906-023-03847-7
Efficacy and safety of Obex® in overweight and obese subjects: a randomised, double-blind, placebo-controlled clinical trial
Abstract
Background: Obex® may be helpful in reducing body weight and fat. The current study was carried out to evaluate the efficacy and safety of Obex® in the treatment of overweight and obese subjects.
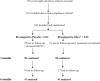
Methods: A double-blind, randomised, controlled phase III clinical trial was conducted involving 160 overweight and obese subjects (BMI ≥ 25.0 and < 40 kg/m2) aged 20 to 60 years, who received Obex® (n = 80) and placebo (n = 80) plus non-pharmacological treatment (physical activity and nutritional counseling). One sachet of Obex® or placebo were administered before the two main meals each day for 6 months. In addition to anthropometric measurements and blood pressure, fasting plasma and 2 h glucose levels during the oral glucose tolerance test, lipid profile, insulin, liver enzymes, creatinine, and uric acid (UA) were determined, insulin resistance (HOMA-IR) beta-cell function (HOMA-β) were assessed and insulin sensitivity (IS) was calculated with three indirect indexes.
Results: After 3 months of Obex®, 48.3% of the participants (28/58) achieved complete success in reducing both weight and waist circumference by greater than or equal to 5% from baseline, as opposed to 26.0% (13/50) of individuals receiving placebo (p = 0.022). Compared to baseline, at 6 months no differences were found between the groups concerning anthropometric and biochemical measurements, except for high-density lipoprotein cholesterol (HDL-c) levels, which were higher in subjects receiving Obex® compared to those receiving placebo (p = 0.030). After 6 months of treatment, both groups showed reduced cholesterol and triglyceride levels (p < 0.012) compared to baseline value. However, only those intake Obex® showed reduced insulin concentrations and HOMA-IR, improved IS (p < 0.05), and decreased creatinine and UA levels (p < 0.005).
Conclusions: The consumption of Obex® together with lifestyle changes increased HDL-c, contributed to a rapid reduction of weight and waist circumference, as well as improved insulin homeostasis, which did not occur in the placebo group, and appears to be safe as an adjunct at conventional obesity treatment.
Trial registration: Clinical trial protocol was registered in the Cuban public registry of clinical trials under code RPCEC00000267 on 17/04/2018 and also registered in the international registry of clinical trials, ClinicalTrials.gov, under code: NCT03541005 on 30/05/2018.
Keywords: Dietary supplementation; Insulin resistance; Obesity; Overweight; Uric acid; Weight loss.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest. The authors are responsible for the content and writing of the article. The funders had no role in the study design, data collection and analysis, decision to publish or manuscript preparation.
Figures
References
-
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects), Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, et al. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383(9921):970–83. - PMC - PubMed
-
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–S138. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

