A pilot randomized controlled trial of ketamine in Borderline Personality Disorder
- PMID: 36804489
- PMCID: PMC10209175
- DOI: 10.1038/s41386-023-01540-4
A pilot randomized controlled trial of ketamine in Borderline Personality Disorder
Abstract
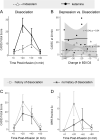
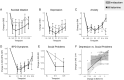
This study is the first randomized controlled trial to test the effects of ketamine in Borderline Personality Disorder (BPD). BPD remains undertreated in the community and no medication has FDA approval for this indication. People with BPD experience chronic mood disturbances with depressed mood, suicidal ideation, and severe social difficulties. In this double-blind, randomized controlled pilot study, we tested the effects of one infusion of ketamine (0.5 mg/kg, n = 10) or the psychoactive comparator drug midazolam (0.04 mg/kg, n = 12) in adults with BPD. Infusions were well tolerated in both groups. Dissociative symptoms during infusion were more intense with ketamine than midazolam (t(12.3) = 3.61, p = 0.01), but they resolved by 40 min after infusion in both groups. Post-infusion adverse events were at the expected low levels in both groups. For our primary outcome measure of suicidal ideation and our secondary outcome measure of depression, we found numerical reduction but not significant group or group x timepoint difference (p > 0.05). For our secondary outcome measures of anxiety and BPD symptoms, we did not observe group or group x timepoint differences. There was a group x timepoint effect for socio-occupational functioning (F(1,20.12) = 5.16, p = 0.03, at Day 14, ketamine group showed more improvement than midazolam group). An exploratory analysis revealed that improvement in socio-occupational functioning was correlated with improvement in depression in the ketamine group (r(8) = 0.65, p = 0.04) but not midazolam group (r(9) = 0.41, p = 0.216). This pilot study provides the first randomized controlled evidence of the effects of antidepressant-dosed ketamine in people with BPD. Our results provide reason for optimism that antidepressant-dosed ketamine will be well-tolerated in larger studies and may provide clinical benefit for mood symptoms and related impairments in people with BPD.
© 2023. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
SKF discloses work with the pharmaceutical company Boehringer Ingelheim as site PI for a multinational clinical trial and for consulting on advisory boards (< $10,000 in 2022). PRC is co-founder of Tetricus Labs. JHK consults for Aptinyx Inc., Biogen Idec MA, Bionomics Ltd., Boehringer Ingelheim International, Clearmind Medicine, Inc., Cybin IRL, Enveric Biosciences, Epiodyne, Inc., EpiVario, Inc., Janssen Research & Development, Jazz Pharmaceuticals, Inc., Otsuka America Pharmaceutical, Inc., Perception Neuroscience, Inc., Praxis Precision Medicines, Inc., Spring Care, Inc., and Sunovion Pharmaceuticals, Inc.; is a scientific advisor of Biohaven Pharmaceuticals, BioXcel Therapeutics, Inc. (Clinical Advisory Board), Cerevel Therapeutics, LLC, Delix Therapeutics, Inc., Eisai, Inc., EpiVario, Inc., Freedom Biosciences, Inc., Jazz Pharmaceuticals, Inc., Numara Therapeutics, Inc., Neurocrine Biosciences, Inc., Novartis Pharmaceuticals Corporation, Perception Neuroscience, Inc., Praxis Precision Medicines, Inc., PsychoGenics, Inc., Takeda Industries, Tempero Bio, Inc., and Terran Biosciences, Inc.; holds stock or stock options with Biohaven Pharmaceuticals, Clearmind Medicine, Inc., Spring Care, Inc., EpiVario, Inc., Neumora Therapeutics, Inc., Tempero Bio, Inc., and Terran Biosciences, Inc.; and is editor of Biological Psychiatry. JHK was also awarded the following patents: (i) Seibyl JP, Krystal JH, Charney DS. Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia. U.S. Patent No. 5447948. September 5, 1995; (ii) Vladimir C, Krystal JH, Sanacora G. Glutamate Modulating Agents in the Treatment of Mental Disorders. U.S. Patent No. 8778979 B2. Patent Issue Date July 15, 2014. U.S. Patent Application No. 15/695,164. Filing Date September 5, 2017; (iii) Charney D, Krystal JH, Manji H, Matthew S, Zarate C. Intranasal Administration of Ketamine to Treat Depression. U.S. Patent Application No. 14/197767 filed on March 5, 2014. U.S. Application or Patent Cooperation Treaty International Application No. 14/306382 filed on June 17, 2014; (iv) Zarate C, Charney DS, Manji HK, Mathew SJ, Krystal JH, Department of Veterans Affairs. Methods for Treating Suicidal Ideation. Patent Application No. 14/197767 filed on March 5, 2014, by Yale University Office of Cooperative Research; (v) Arias A, Petrakis I, Krystal JH. Composition and Methods to Treat Addiction. Provisional Use Patent Application No. 61/973/961. April 2, 2014. Filed by Yale University Office of Cooperative Research; (vi) Chekroud A, Gueorguieva R, Krystal JH. Treatment Selection for Major Depressive Disorder. U.S. Patent and Trademark Office Docket No. Y0087.70116US00. Filed June 3, 2016. Provisional patent submission by Yale University; (vii) Gihyun Y, Petrakis I, Krystal JH. Compounds, Compositions, and Methods for Treating or Preventing Depression and Other Diseases. U.S. Provisional Patent Application No. 62/444552. Filed on January 10, 2017 by Yale University Office of Cooperative Research OCR 7088 US01; (viii) Abdallah C, Krystal JH, Duman R, Sanacora G. Combination Therapy for Treating or Preventing Depression or Other Mood Diseases. U.S. Provisional Patent Application No. 62/719935. Filed on August 20, 2018, by Yale University Office of Cooperative Research OCR 7451 US01. (ix) Krystal, JH, Pearlson, G, O’Malley, S, Potenza, M, Gasparini, F, Gomez-Mancilla, B, Malaterre, V. Mavoglurant in treating gambling and gaming disorders. U.S. Provisional Patent Application No. 63/125,181filed on December 14, 2020 by Yale University Office of Cooperative Research OCR 8065 US00. AstraZeneca Pharmaceuticals provides the drug, Saracatinib, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-5]”. Novartis provides the drug, Mavoglurant, for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-5]”. Cerevel provides the drug PF-06412562 for A Translational and Neurocomputational Evaluation of a D1R Partial Agonist for Schizophrenia (1 U01 MH121766-01). EYC, RST, KD, EN, MS, KN, DTD, JR, GFP, and JRP declare no competing interests.
Figures
References
-
- Binks CA, Fenton M, McCarthy L, Lee T, Adams CE, Duggan C. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. 2006:CD005653. 10.1002/14651858.CD005653 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

