Pathways of MHC I cross-presentation of exogenous antigens
- PMID: 36804685
- PMCID: PMC10023513
- DOI: 10.1016/j.smim.2023.101729
Pathways of MHC I cross-presentation of exogenous antigens
Abstract
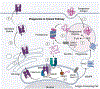
Phagocytes, particularly dendritic cells (DCs), generate peptide-major histocompatibility complex (MHC) I complexes from antigens they have collected from cells in tissues and report this information to CD8 T cells in a process called cross-presentation. This process allows CD8 T cells to detect, respond and eliminate abnormal cells, such as cancers or cells infected with viruses or intracellular microbes. In some settings, cross-presentation can help tolerize CD8 T cells to self-antigens. One of the principal ways that DCs acquire tissue antigens is by ingesting this material through phagocytosis. The resulting phagosomes are key hubs in the cross-presentation (XPT) process and in fact experimentally conferring the ability to phagocytize antigens can be sufficient to allow non-professional antigen presenting cells (APCs) to cross-present. Once in phagosomes, exogenous antigens can be cross-presented (XPTed) through three distinct pathways. There is a vacuolar pathway in which peptides are generated and then bind to MHC I molecules within the confines of the vacuole. Ingested exogenous antigens can also be exported from phagosomes to the cytosol upon vesicular rupture and/or possibly transport. Once in the cytosol, the antigen is degraded by the proteasome and the resulting oligopeptides can be transported to MHC I molecule in the endoplasmic reticulum (ER) (a phagosome-to-cytosol (P2C) pathway) or in phagosomes (a phagosome-to-cytosol-to-phagosome (P2C2P) pathway). Here we review how phagosomes acquire the necessary molecular components that support these three mechanisms and the contribution of these pathways. We describe what is known as well as the gaps in our understanding of these processes.
Keywords: Antigen presentation; Cross-presentation; MHC I; Phagosome.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest None.
Figures
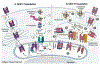


References
-
- Bevan MJ, Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming, J. Immunol 117 (6) (1976) 2233–2238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

