Regulatory T Cell Amelioration of Graft-versus-Host Disease following Allogeneic/Xenogeneic Hematopoietic Stem Cell Transplantation Using Mobilized Mouse and Human Peripheral Blood Donors
- PMID: 36804930
- PMCID: PMC10149591
- DOI: 10.1016/j.jtct.2023.02.015
Regulatory T Cell Amelioration of Graft-versus-Host Disease following Allogeneic/Xenogeneic Hematopoietic Stem Cell Transplantation Using Mobilized Mouse and Human Peripheral Blood Donors
Abstract
The present studies examined experimental transplant outcomes using mobilized peripheral blood from mice and humans together with FoxP3+Treg cells. Donor mice were treated with filgrastim and / or plerixafor and their peripheral blood (PB) displayed significant elevations in hematopoietic stem and progenitor populations. Some of these PB donors were concurrently administered a Treg expansion strategy consisting of a TL1A-Ig fusion protein low dose rIL-2. A significant increase (4-5x) in the frequency Tregs occurred during mobilization. C3H.SW PB was collected from mobilized and Treg unexpanded ("TrUM") or mobilized and Treg expanded ("TrEM") donors and transplanted into MHC-matched B6 (H2b) recipients. Recipients of TrEM, exhibited significantly reduced weight loss and clinical GVHD scores compared to recipients of TrUM. Notably, recipients of TrEM exhibited comparable GVL activity to TrUM recipients against leukemia levels. Next, huTregs (CD4+CD25+CD127lo) from a healthy human PB mobilized donor were expanded ex-vivo prior to transplant into NSG/ NOD-scid IL2Rgammanull mice. We found that treatment with ex-vivo expanded huTregs resulted in significant reduction of lethality and clinical xGVHD scores. Notably, post-transplant, PB huTregs levels remained elevated and the frequency of huCD4+Tconv and CD8+ cells was diminished supporting the improved xGVHD outcomes. These findings demonstrated that the use of mPB containing elevated Treg levels significantly reduced GVHD following "MUD" and MHC-mismatched mouse HSCT without loss of GVL activity. Moreover, utilizing ex-vivo expanded huTregs from a mobilized PB donor and added back to donor PB ameliorated xGVHD. In total, these studies support the notion that in vivo or ex-vivo manipulation of donor Tregs together with mobilized peripheral blood could provide therapeutic approaches to improve aHSCT outcomes.
Keywords: Allogeneic Hematopoietic Stem Cell Transplantation; GVT, Xenotransplantation; Graft-versus-Host Disease; Mobilization; TL1A; Tregs, IL-2.
Copyright © 2023 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
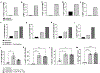
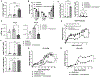

Similar articles
-
BET Bromodomain Inhibitors Which Permit Treg Function Enable a Combinatorial Strategy to Suppress GVHD in Pre-clinical Allogeneic HSCT.Front Immunol. 2019 Jan 24;9:3104. doi: 10.3389/fimmu.2018.03104. eCollection 2018. Front Immunol. 2019. PMID: 30733722 Free PMC article.
-
Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation.Biol Blood Marrow Transplant. 2017 May;23(5):757-766. doi: 10.1016/j.bbmt.2017.02.013. Epub 2017 Feb 20. Biol Blood Marrow Transplant. 2017. PMID: 28219835 Free PMC article.
-
Mobilized Multipotent Hematopoietic Progenitors Promote Expansion and Survival of Allogeneic Tregs and Protect Against Graft Versus Host Disease.Front Immunol. 2021 Feb 12;11:607180. doi: 10.3389/fimmu.2020.607180. eCollection 2020. Front Immunol. 2021. PMID: 33643294 Free PMC article.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation.Blood Cells Mol Dis. 2008 Jan-Feb;40(1):48-54. doi: 10.1016/j.bcmd.2007.06.019. Epub 2007 Sep 10. Blood Cells Mol Dis. 2008. PMID: 17827036 Review.
Cited by
-
Pathophysiology and preclinical relevance of experimental graft-versus-host disease in humanized mice.Biomark Res. 2024 Nov 14;12(1):139. doi: 10.1186/s40364-024-00684-9. Biomark Res. 2024. PMID: 39543777 Free PMC article. Review.
-
Immune Monitoring after Cell Therapy and Hematopoietic Cell Transplantation: Guidelines by the ISCT Stem Cell Engineering Committee.Cytotherapy. 2025 Aug;27(8):888-902. doi: 10.1016/j.jcyt.2025.04.069. Epub 2025 May 5. Cytotherapy. 2025. PMID: 40493000 Free PMC article. Review.
-
The impact of regulatory T cells on the graft-versus-leukemia effect.Front Immunol. 2024 Apr 22;15:1339318. doi: 10.3389/fimmu.2024.1339318. eCollection 2024. Front Immunol. 2024. PMID: 38711496 Free PMC article. Review.
References
-
- Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood 105 (2005) 1828–36. - PubMed
-
- Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR, and Wagner JE, Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 127 (2016) 1044–51. - PMC - PubMed
-
- Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velard Ai, Aversa F, Martelli MF, Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117 (2011) 3921–8. - PubMed
-
- Wolf D, Barreras H, Bader CS, Copsel SN, Lightbourn CO, Pfeiffer BJ, Altman NH, Podack ER, Komanduri KV, and Levy RB. Marked in Vivo Donor Regulatory T Cell Expansion via Interleukin-2 and TL1A-Ig Stimulation Ameliorates Graft-versus-Host Disease but Preserves Graft-versus-Leukemia in Recipients after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 23 (2017) 757–766. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

