MMG22 Potently Blocks Hyperalgesia in Cisplatin-treated Mice
- PMID: 36805004
- PMCID: PMC10065962
- DOI: 10.1016/j.neuroscience.2023.02.006
MMG22 Potently Blocks Hyperalgesia in Cisplatin-treated Mice
Abstract
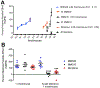
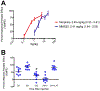
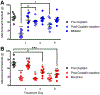
MMG22 is a bivalent ligand containing MOR agonist and mGluR5 antagonist pharmacophores connected by a 22-atom linker. Intrathecal (i.t.) administration of MMG22 to inflamed mice has been reported to produce fmol-range antinociception in the reversal of LPS-induced hyperalgesia. MMG22 reduced hyperalgesia in the spared nerve injury (SNI) model of neuropathic pain at 10 days after injury but not at 30 days after injury, perhaps related to the inflammation that occurs early after injury but subsequently subsides. The present study determined the efficacy of MMG22 in cisplatin-treated male mice in order to provide data relating to the efficacy of MMG22 in the treatment of neuropathic pain that is associated with inflammation. Groups of eight mice each received daily intraperitoneal (i.p.) injections of cisplatin for seven days to produce robust mechanical allodynia defined by the decrease in withdrawal threshold using an electronic von Frey applied to the plantar surface of the hind paw. Intrathecal administration of MMG22 potently reduced mechanical hyperalgesia (ED50 0.04 fmol/mouse) without tolerance, whereas MMG10 was essentially inactive. Morphine was less potent than MMG22 by >5-orders of magnitude and displayed tolerance. Subcutaneous MMG22 was effective (ED50 = 2.41 mg/kg) and devoid of chronic tolerance. We propose that MMG22 induces the formation of a MOR-mGluR5 heteromer through selective interaction with the upregulated NR2B subunit of activated NMDAR, in view of the 4600-fold reduction of i.t. MMG22 antinociception by the selective NR2B antagonist, Ro25-6981. A possible explanation for the substantially reduced potency for MMG22 in the SNI model is discussed.
Keywords: MMG22; antihyperalgesia; antinociception; cisplatin; neuropathic pain.
Copyright © 2023 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare no competing financial interest.
Figures
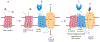
Similar articles
-
Targeting MOR-mGluR5 heteromers reduces bone cancer pain by activating MOR and inhibiting mGluR5.Neuropharmacology. 2019 Dec 1;160:107690. doi: 10.1016/j.neuropharm.2019.107690. Epub 2019 Jul 1. Neuropharmacology. 2019. PMID: 31271770 Free PMC article.
-
Bivalent ligand that activates mu opioid receptor and antagonizes mGluR5 receptor reduces neuropathic pain in mice.Pain. 2017 Dec;158(12):2431-2441. doi: 10.1097/j.pain.0000000000001050. Pain. 2017. PMID: 28891868 Free PMC article.
-
The bivalent ligand, MMG22, reduces neuropathic pain after nerve injury without the side effects of traditional opioids.Pain. 2020 Sep 1;161(9):2041-2057. doi: 10.1097/j.pain.0000000000001902. Pain. 2020. PMID: 32345918 Free PMC article.
-
Targeting putative mu opioid/metabotropic glutamate receptor-5 heteromers produces potent antinociception in a chronic murine bone cancer model.Eur J Pharmacol. 2014 Nov 15;743:48-52. doi: 10.1016/j.ejphar.2014.09.008. Epub 2014 Sep 17. Eur J Pharmacol. 2014. PMID: 25239072 Free PMC article.
-
Combined Glia Inhibition and Opioid Receptor Agonism Afford Highly Potent Analgesics without Tolerance.ACS Chem Neurosci. 2019 Apr 17;10(4):2004-2011. doi: 10.1021/acschemneuro.8b00323. Epub 2018 Aug 24. ACS Chem Neurosci. 2019. PMID: 30110531
References
-
- Akgün E, Lunzer MM, Portoghese PS (2019) Combined glia inhibition and opioid receptor agonism afford highly potent analgesics without tolerance. ACS Chem Neurosci 10:2004–2011. - PubMed
-
- Bleakman D, Rusin KI, Chard PS, Glaum SR, Miller RJ (1992) Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol Pharmacol 43:192–196. - PubMed
-
- Boxall SJ, Berthele A, Tölle TR, Zieglgänsberger W, Urban L (1998) mGluR activation reveals a tonic NMDA component in inflammatory hyperalgesia. Neuroreport 9:1201–1203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

