TREM2 splice isoforms generate soluble TREM2 species that disrupt long-term potentiation
- PMID: 36805764
- PMCID: PMC9940368
- DOI: 10.1186/s13073-023-01160-z
TREM2 splice isoforms generate soluble TREM2 species that disrupt long-term potentiation
Abstract
Background: TREM2 is a transmembrane receptor expressed by myeloid cells and acts to regulate their immune response. TREM2 governs the response of microglia to amyloid and tau pathologies in the Alzheimer's disease (AD) brain. TREM2 is also present in a soluble form (sTREM2), and its CSF levels fluctuate as a function of AD progression. Analysis of stroke and AD mouse models revealed that sTREM2 proteins bind to neurons, which suggests sTREM2 may act in a non-cell autonomous manner to influence neuronal function. sTREM2 arises from the proteolytic cleavage of the membrane-associated receptor. However, alternatively spliced TREM2 species lacking a transmembrane domain have been postulated to contribute to the pool of sTREM2. Thus, both the source of sTREM2 species and its actions in the brain remain unclear.
Methods: The expression of TREM2 isoforms in the AD brain was assessed through the analysis of the Accelerating Medicines Partnership for Alzheimer's Disease Consortium transcriptomics data, as well as qPCR analysis using post-mortem samples of AD patients and of the AD mouse model 5xFAD. TREM2 cleavage and secretion were studied in vitro using HEK-293T and HMC3 cell lines. Synaptic plasticity, as evaluated by induction of LTP in hippocampal brain slices, was employed as a measure of sTREM2 actions.
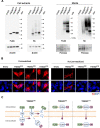
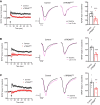
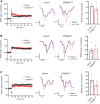
Results: Three distinct TREM2 transcripts, namely ENST00000373113 (TREM2230), which encodes the full-length transmembrane receptor, and the alternatively spliced isoforms ENST00000373122 (TREM2222) and ENST00000338469 (TREM2219), are moderately increased in specific brain regions of patients with AD. We provide experimental evidence that TREM2 alternatively spliced isoforms are translated and secreted as sTREM2. Furthermore, our functional analysis reveals that all sTREM2 species inhibit LTP induction, and this effect is abolished by the GABAA receptor antagonist picrotoxin.
Conclusions: TREM2 transcripts can give rise to a heterogeneous pool of sTREM2 which acts to inhibit LTP. These results provide novel insight into the generation, regulation, and function of sTREM2 which fits into the complex biology of TREM2 and its role in human health and disease. Given that sTREM2 levels are linked to AD pathogenesis and progression, our finding that sTREM2 species interfere with LTP furthers our understanding about the role of TREM2 in AD.
Keywords: Alzheimer’s disease; Soluble TREM2; TREM2; TREM2 splicing.
© 2023. The Author(s).
Conflict of interest statement
The Indiana CTSI Eli Lilly-Stark Neurosciences Post-Doctoral Research Fellowship in Neurodegeneration does not constitute a competing interest. The authors declare that they have no competing interests.
Figures


References
-
- Sims R, van der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, Kunkle BW, Boland A, Raybould R, Bis JC, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

