N-glycolylneuraminic acid as a carbohydrate cancer biomarker
- PMID: 36805917
- PMCID: PMC9971276
- DOI: 10.1016/j.tranon.2023.101643
N-glycolylneuraminic acid as a carbohydrate cancer biomarker
Abstract
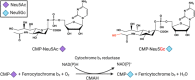
One of the forms of aberrant glycosylation in human tumors is the expression of N-glycolylneuraminic acid (Neu5Gc). The only known enzyme to biosynthesize Neu5Gc in mammals, cytidine-5'-monophosphate-N-acetylneuraminic acid (CMAH), appears to be genetically inactivated in humans. Regardless, low levels of Neu5Gc have been detected in healthy humans. Therefore, it is proposed that the presence of Neu5Gc in humans is from dietary acquisition, such as red meat. Notably, detection of elevated Neu5Gc levels has been repeatedly found in cancer tissues, cells and serum samples, thereby Neu5Gc-containing antigens may be exploited as a class of cancer biomarkers. Here we review the findings to date on using Neu5Gc-containing tumor glycoconjugates as a class of cancer biomarkers for cancer detection, surveillance, prognosis and therapeutic targets. We review the evidence that supports an emerging hypothesis of de novo Neu5Gc biosynthesis in human cancer cells as a source of Neu5Gc in human tumors, generated under certain metabolic conditions.
Keywords: Cancer biomarker; Detection; N-glycolylneuraminic acid; Neu5Gc; Prognosis.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The following authors (CJD and MPJ) declare that they are named inventors on a patent on the SubB2M technology (WO2018085888A1) and a second patent (CJD, LKS and MPJ) for improvements to the SubB2M test (2,021,901,444). Both of these patents were licensed to Inoviq, VIC, Australia in 2020.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

