Evolutionary origins and interactomes of human, young microproteins and small peptides translated from short open reading frames
- PMID: 36806354
- PMCID: PMC10032668
- DOI: 10.1016/j.molcel.2023.01.023
Evolutionary origins and interactomes of human, young microproteins and small peptides translated from short open reading frames
Abstract
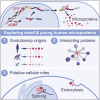
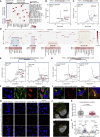
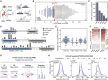
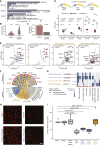
All species continuously evolve short open reading frames (sORFs) that can be templated for protein synthesis and may provide raw materials for evolutionary adaptation. We analyzed the evolutionary origins of 7,264 recently cataloged human sORFs and found that most were evolutionarily young and had emerged de novo. We additionally identified 221 previously missed sORFs potentially translated into peptides of up to 15 amino acids-all of which are smaller than the smallest human microprotein annotated to date. To investigate the bioactivity of sORF-encoded small peptides and young microproteins, we subjected 266 candidates to a mass-spectrometry-based interactome screen with motif resolution. Based on these interactomes and additional cellular assays, we can associate several candidates with mRNA splicing, translational regulation, and endocytosis. Our work provides insights into the evolutionary origins and interaction potential of young and small proteins, thereby helping to elucidate this underexplored territory of the human proteome.
Keywords: PRISMA; de novo genes; microproteins; primate-specific proteins; protein evolution; protein interactome; ribosome profiling; short ORFs; short linear motifs, SLiMs; short peptides.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

