Copy number variation-associated lncRNAs may contribute to the etiologies of congenital heart disease
- PMID: 36806749
- PMCID: PMC9938258
- DOI: 10.1038/s42003-023-04565-z
Copy number variation-associated lncRNAs may contribute to the etiologies of congenital heart disease
Abstract
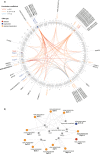
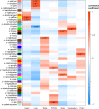
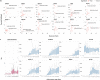
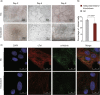
Copy number variations (CNVs) have long been recognized as pathogenic factors for congenital heart disease (CHD). Few CHD associated CNVs could be interpreted as dosage effect due to disruption of coding sequences. Emerging evidences have highlighted the regulatory roles of long noncoding RNAs (lncRNAs) in cardiac development. Whereas it remains unexplored whether lncRNAs within CNVs (CNV-lncRNAs) could contribute to the etiology of CHD associated CNVs. Here we constructed coexpression networks involving CNV-lncRNAs within CHD associated CNVs and protein coding genes using the human organ developmental transcriptomic data, and showed that CNV-lncRNAs within 10 of the non-syndromic CHD associated CNVs clustered in the most significant heart correlated module, and had highly correlated coexpression with multiple key CHD genes. HSALNG0104472 within 15q11.2 region was identified as a hub CNV-lncRNA with heart-biased expression and validated experimentally. Our results indicated that HSALNG0104472 should be a main effector responsible for cardiac defects of 15q11.2 deletion through regulating cardiomyocytes differentiation. Our findings suggested that CNV-lncRNAs could potentially contribute to the pathologies of a maximum proportion of 68.4% (13/19) of non-syndromic CHD associated CNVs. These results indicated that explaining the pathogenesis of CHD associated CNVs should take account of the noncoding regions.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

