Deep haplotype analyses of target-site resistance locus ACCase in blackgrass enabled by pool-based amplicon sequencing
- PMID: 36807472
- PMCID: PMC10214753
- DOI: 10.1111/pbi.14033
Deep haplotype analyses of target-site resistance locus ACCase in blackgrass enabled by pool-based amplicon sequencing
Abstract
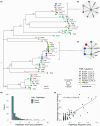
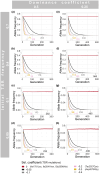
Rapid adaptation of weeds to herbicide applications in agriculture through resistance development is a widespread phenomenon. In particular, the grass Alopecurus myosuroides is an extremely problematic weed in cereal crops with the potential to manifest resistance in only a few generations. Target-site resistances (TSRs), with their strong phenotypic response, play an important role in this rapid adaptive response. Recently, using PacBio's long-read amplicon sequencing technology in hundreds of individuals, we were able to decipher the genomic context in which TSR mutations occur. However, sequencing individual amplicons are costly and time-consuming, thus impractical to implement for other resistance loci or applications. Alternatively, pool-based approaches overcome these limitations and provide reliable allele frequencies, although at the expense of not preserving haplotype information. In this proof-of-concept study, we sequenced with PacBio High Fidelity (HiFi) reads long-range amplicons (13.2 kb), encompassing the entire ACCase gene in pools of over 100 individuals, and resolved them into haplotypes using the clustering algorithm PacBio amplicon analysis (pbaa), a new application for pools in plants and other organisms. From these amplicon pools, we were able to recover most haplotypes from previously sequenced individuals of the same population. In addition, we analysed new pools from a Germany-wide collection of A. myosuroides populations and found that TSR mutations originating from soft sweeps of independent origin were common. Forward-in-time simulations indicate that TSR haplotypes will persist for decades even at relatively low frequencies and without selection, highlighting the importance of accurate measurement of TSR haplotype prevalence for weed management.
Keywords: ACCase; Alopecurus myosuroides; pbaa; Amplicon sequencing; HiFi long reads; herbicide resistance.
© 2023 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
J.H. is the founder and M.H. is the owner of Agris42, a company providing herbicide resistance testing services and weed management consultation to farmers. D.W. holds equity and S.K. is an employee of Computomics, which advises breeders. Z.N.K. is an employee and shareholder of Pacific Biosciences, a company developing single molecule‐sequencing technologies. Other authors declare no competing or financial interest.
Figures



References
-
- Anthimidou, E. , Ntoanidou, S. , Madesis, P. and Eleftherohorinos, I. (2020) Mechanisms of Lolium rigidum multiple resistance to ALS‐ and ACCase‐inhibiting herbicides and their impact on plant fitness. Pestic. Biochem. Physiol. 164, 65–72. - PubMed
-
- Anthony, R.G. , Waldin, T.R. , Ray, J.A. , Bright, S.W. and Hussey, P.J. (1998) Herbicide resistance caused by spontaneous mutation of the cytoskeletal protein tubulin. Nature 393, 260–263. - PubMed
-
- Beckie, H.J. and Tardif, F.J. (2012) Herbicide cross resistance in weeds. Crop Prot. 35, 15–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

