Biomimetic Bilayered Scaffolds for Tissue Engineering: From Current Design Strategies to Medical Applications
- PMID: 36807830
- PMCID: PMC11469754
- DOI: 10.1002/adhm.202203115
Biomimetic Bilayered Scaffolds for Tissue Engineering: From Current Design Strategies to Medical Applications
Abstract
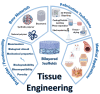
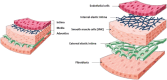
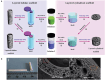
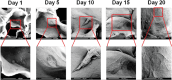
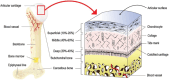
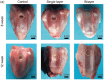
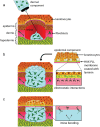
Tissue damage due to cancer, congenital anomalies, and injuries needs new efficient treatments that allow tissue regeneration. In this context, tissue engineering shows a great potential to restore the native architecture and function of damaged tissues, by combining cells with specific scaffolds. Scaffolds made of natural and/or synthetic polymers and sometimes ceramics play a key role in guiding cell growth and formation of the new tissues. Monolayered scaffolds, which consist of uniform material structure, are reported as not being sufficient to mimic complex biological environment of the tissues. Osteochondral, cutaneous, vascular, and many other tissues all have multilayered structures, therefore multilayered scaffolds seem more advantageous to regenerate these tissues. In this review, recent advances in bilayered scaffolds design applied to regeneration of vascular, bone, cartilage, skin, periodontal, urinary bladder, and tracheal tissues are focused on. After a short introduction on tissue anatomy, composition and fabrication techniques of bilayered scaffolds are explained. Then, experimental results obtained in vitro and in vivo are described, and their limitations are given. Finally, difficulties in scaling up production of bilayer scaffolds and reaching the stage of clinical studies are discussed when multiple scaffold components are used.
Keywords: bilayered scaffolds; biomaterials; biomimetism; material design; medical applications; tissue engineering.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

